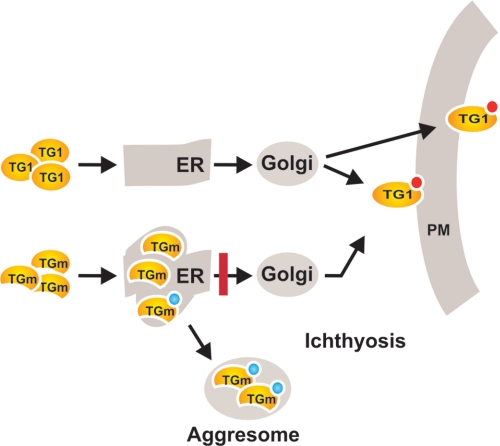
FIGURE 10.
Schematic summary of transglutaminase trafficking. We propose that TG1 is synthesized and trafficked via the ER (and probably the Golgi) for export to the cell exterior and incorporation at the inner surface of the plasma membrane. Our EM and confocal imaging, differential membrane extraction, and proteinase challenge studies suggest that TG1 is associated on the inner surface of the ER lumen. Previous studies show that TG1 is myristoylated and palmitoylated (red circle) and suggest that this is required for TG1 interaction with membranes (39, 40, 44). We are not sure that exported TG1 is myristoylated. In contrast, misfolded TG1 mutants (TGm) are trapped (red bar) in the ER and are ubiquitinylated. Some of this protein is not efficiently removed by proteasome processing and accumulates in aggresomes. We further propose that ER and aggresome accumulation of mutant TG (TGm) contributes to the ichthyosis disease phenotype via two complimentary mechanisms: reducing TG1 level and activity, and activating other cell responses (e.g. ER-associated protein degradation response (63)).