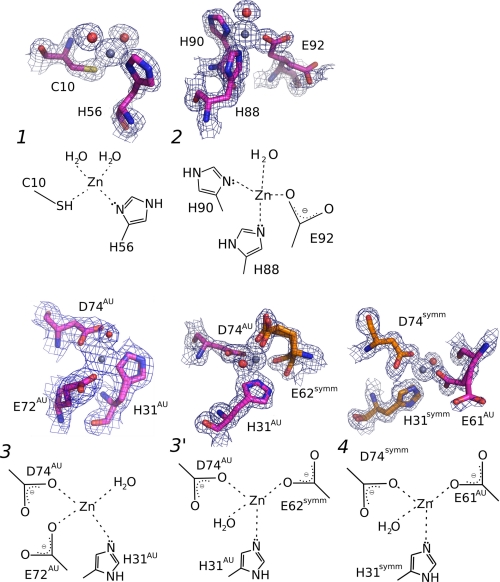
FIGURE 2.
Details of the three Zn2+-binding sites in TTR (ZBS 1–3), and a fourth site (ZBS 4) that is formed by amino acid residues from a symmetry-related tetramer. Close-up views of Zn2+-binding sites and schematic diagrams of Zn2+ coordination spheres. ZBS 1 is composed of Cys-10, His-56, and two water molecules (red spheres). ZBS 2 is composed of His-88, His-90, Glu-92, and one water molecule. ZBS 3 involves His31AU, Asp74AU and Glu72AU (at higher pH values) or Glu62symm (at pH4.6, ZBS 3′). ZBS 4 involves His31symm, Asp74symm, and Glu61AU in addition to water molecules (AU, asymmetric unit; symm, symmetry-related unit). Meshes are electron densities from 2 Fobs-Fcalc omit maps contoured at 1σ. The electron density displayed is limited to within 1.5 Å of the residues. Orange residues in ZBS 3 and ZBS 4 represent residues from neighboring tetramers of symmetrically related molecules in the crystal. The Zn2+-binding sites described here present the canonical constitution of other selective Zn2+-binding sites found in proteins (40).