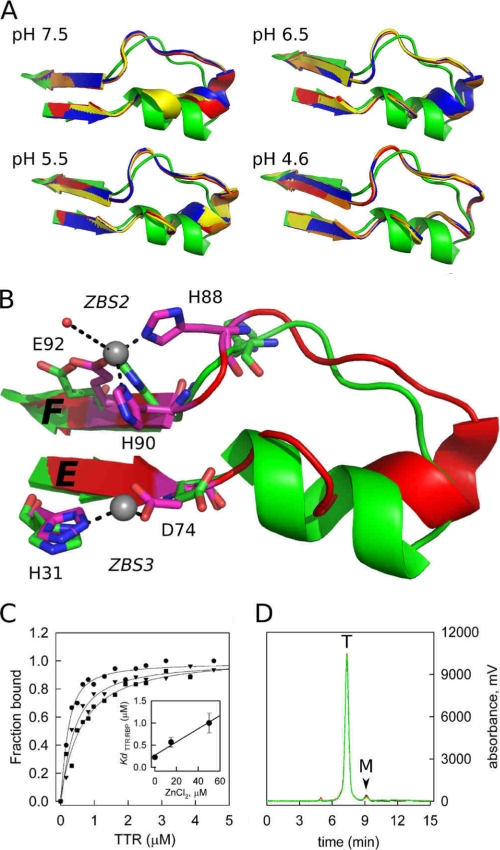
FIGURE 3.
A close-up view of the loop E-loop F region flanked by ZBS 2 and ZBS 3 shows major structural modifications when Zn2+ is bound. A, structures of the four subunits of TTR (ABCD; orange, red, blue, and yellow) obtained in the presence of Zn2+ at different pH are overlapped with the Zn2+-free structure (green). Note the movement of the α-helix residues as the pH decreases from pH 7.5 to 4.6, causing the helix to unwind and form an extended loop. B, close-up view of the ZBS 2 and 3 region, in green, the apo form and in red the structure obtained in the presence of Zn2+ at pH 7.5, where the structural alterations in the α-helix region are better seen. Residues in pink are those that undergo major reorientation upon Zn2+ binding. Zn2+ atoms are shown as gray spheres. C, Zn2+ binding to WT-TTR decreases its affinity for holo-RBP. Isothermal holo-RBP:WT-TTR binding assays were performed by measuring anisotropy changes that take place upon TTR binding to holo-RBP in the absence (●) or in the presence of 15 μm ZnCl2 (▼) and 50 μm ZnCl2 (■). Data are normalized to fraction of holo-RBP:TTR complex formed. The Kd values (inset, error bars show ± S.D.) were calculated as described under “Experimental Procedures.” D, size-exclusion chromatography demonstrates that WT-TTR remains a tetramer upon Zn2+ binding. The elution profiles of WT-TTR without (black line) and in the presence of 20 μm (red line) and 100 μm (green line) ZnCl2 are shown. T, elution time of tetrameric TTR; M, elution time of monomeric TTR.