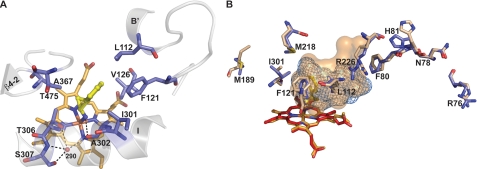
FIGURE 4.
Views of the CYP46A1 active site illustrating interactions with TCP. A, residues in contact with TCP are shown. Portions of the secondary structure are also shown. B, superposition of TCP-bound (slate) versus ligand-free (wheat) CYP46A1 show the active site volumes and amino acid residues that undergo conformational changes upon TCP binding. The heme group in TCP and ligand-free CYP46A1 is in light orange and red, respectively, and TCP is in yellow. The nitrogen, oxygen, sulfur, and iron atoms are in blue, red, yellow, and orange, respectively. H2O molecule 290 is shown as a red sphere. Dashed black lines indicate hydrogen bonds. Protein side chains are shown as sticks, and the solvent-occupied surface of the active site is shown as a light blue mesh (TCP-bound CYP46A1) and wheat surface (ligand-free CYP46A1).