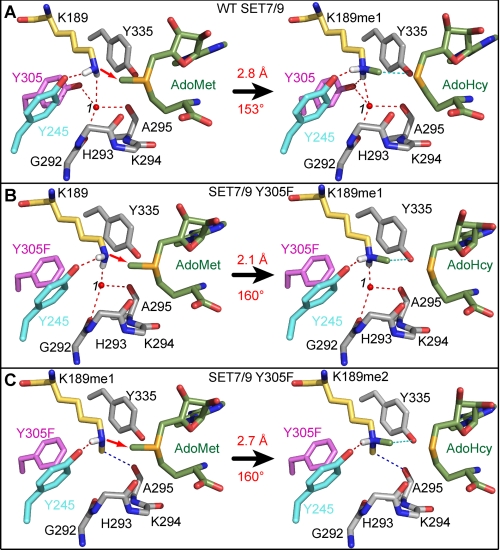
FIGURE 4.
Catalytic models of the methyl transfer reactions catalyzed by WT SET7/9 and the Y305F mutant. A, monomethylation of TAF10-K189 by the WT enzyme. The reaction scheme depicts the modeled substrate ternary complex (left) and the product complex (right) for the transfer of the methyl group from AdoMet (green carbon atoms) to K189 in TAF10 (yellow carbons), yielding AdoHcy and K189me1. The red arrow indicates the direction of the nucleophilic attack of the deprotonated ϵ-amino group on the AdoMet methyl group. The transferred methyl group is colored green, and the white atoms represent the hydrogens of the ϵ-amino group. Hydrogen bonds and residues in the enzyme active site are illustrated as in Fig. 2. The reaction distance and angle are labeled in red. B and C, models of the Y305F mutant for the first methyl transfer reaction with TAF10-K189 (B) and second methyl transfer reaction with TAF10-K189me1 (C). Color schemes are the same as in A.