Table 3. Apparent thermodynamic parameters for the equilibrium unfolding of RaPrPC(121–228) and the I214V mutant at 25°C.
| Protein |
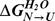 (kJ·mol−1) (kJ·mol−1) |
 (kJ·mol−1·M−1) (kJ·mol−1·M−1) |
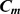 (M) (M) |
| RaPrPC(121–228) | 26.2±2.7 | −3.88±0.49 | 6.49±0.05 |
| I214V | 16.1±1.8 | −2.62±0.38 | 5.65±0.06 |
Note: 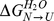 is an estimate of the free energy in the absence of denaturant, the parameter
is an estimate of the free energy in the absence of denaturant, the parameter  represents the cooperativity of the unfolding transition, and
represents the cooperativity of the unfolding transition, and 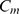 is the concentration of urea at the midpoint of unfolding. The determined parameters for the wild-type [30] are listed here to facilitate comparison.
is the concentration of urea at the midpoint of unfolding. The determined parameters for the wild-type [30] are listed here to facilitate comparison.
