Abstract
Amyloidogenic processing of the amyloid precursor protein (APP) generates a large secreted ectodomain fragment (APPsβ), β-amyloid (Aβ) peptides, and an APP intracellular domain (AICD). Whereas Aβ is viewed as critical for Alzheimer's disease pathogenesis, the role of other APP processing products remains enigmatic. Of interest, the AICD has been implicated in transcriptional regulation, and N-terminal cleavage of APPsβ has been suggested to produce an active fragment that may mediate axonal pruning and neuronal cell death. We previously reported that mice deficient in APP and APP-like protein 2 (APLP2) exhibit early postnatal lethality and neuromuscular synapse defects, whereas mice with neuronal conditional deletion of APP and APLP2 are viable. Using transcriptional profiling, we now identify transthyretin (TTR) and Klotho as APP/APLP2-dependent genes whose expression is decreased in loss-of-function states but increased in gain-of-function states. Significantly, by creating an APP knockin allele that expresses only APPsβ protein, we demonstrate that APPsβ is not normally cleaved in vivo and is fully capable of mediating the APP-dependent regulation of TTR and Klotho gene expression. Despite being an active regulator of gene expression, APPsβ did not rescue the lethality and neuromuscular synapse defects of APP and APLP2 double-KO animals. Our studies identify TTR and Klotho as physiological targets of APP that are regulated by soluble APPsβ independent of developmental APP functions. This unexpected APP-mediated signaling pathway may play an important role in maintaining TTR and Klotho levels and their respective functions in Aβ sequestration and aging.
Keywords: Alzheimer's disease, β-secretase, neuromuscular synapse, knockin, conditional knockout
Amyloid precursor protein (APP) is a ubiquitously expressed type I membrane protein existing either as a full-length protein or as various proteolytically cleaved fragments. In particular, extracellular processing by α- or β-secretase, followed by presenilin-dependent γ-secretase cleavage, generates large soluble derivatives termed α-secretase cleaved soluble APP (APPsα) and β-secretase cleaved soluble APP (APPsβ), respectively; p3 and β-amyloid (Aβ) peptides, and the APP intracellular domain (AICD) (reviewed in ref. 1). Whereas APPsα has been shown to exert neurotrophic and synaptogenic activities, APPsβ was reported to be much less active in these in vitro assays, or may even have inhibitory effects (reviewed in ref. 2). Of interest, a recent publication showed that soluble APPsβ (but not APPsα) undergoes further cleavage to produce an N-terminal APP derivative (N-APP), which binds to DR6 receptor and mediates axon pruning and degeneration under trophic withdrawal conditions (3). The AICD has been proposed to function as a transcriptional regulator but not as a transcription factor, by activating Fe65 and the chromatin-remodeling factor Tip60 (4, 5). Follow-up investigations have identified multiple putative downstream targets (6–11). However, the validity of these proposed targets has been questioned or disputed (12–17).
APP is a member of a conserved protein family that includes APL-1 in Caenorhabditis elegans, and APP, amyloid precursor-like protein 1 (APLP1), and APLP2 in mammals. Whereas APP single-KO mice are viable with only subtle cognitive and motor impairments (18, 19), mice doubly deficient in APP and APLP2 [double-KO (dKO) mice] die soon after birth and exhibit profound neuromuscular junction (NMJ) defects (20, 21). Interestingly, although the intracellular sequences are most highly conserved among the APP family of proteins (reviewed in ref. 22), phenotypes reported in APP-deficient mice have been shown to be reversed by expressing only APPsα (23). The dispensable role of the membrane and intracellular sequences is also corroborated by Hornsten et al. (24), who showed that the lethality of C. elegans mutant deleting apl-1 can be rescued by neuronal expression of soluble APL-1 extracellular domain.
By creating two APP alleles in mice (1), a conditional KO allele with specific APP deletion in neurons and (2) a knockin (ki) allele that expresses only APPsβ, we report here that transthyretin (TTR) and Klotho are physiological targets of APP whose expressions are mediated by APPsβ. Despite the positive regulation in gene expression, APPsβ cannot rescue the developmental phenotypes of the APP/APLP2-deficient mice.
Results
Generation of APPsβ ki Mice and Biochemical Characterization of APPsβ Protein.
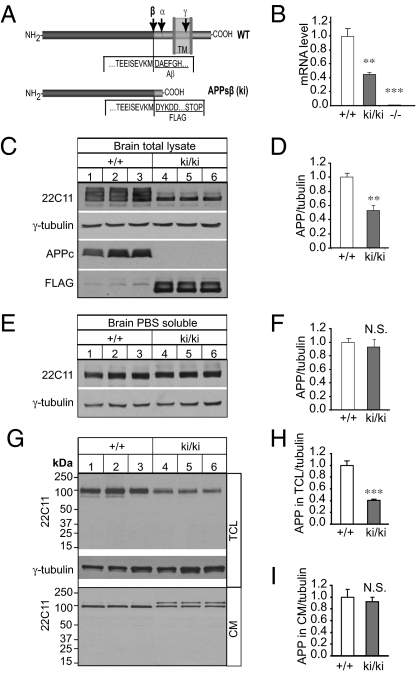
We created an APP ki allele in which a FLAG tag and a stop codon were inserted before the Aβ sequence, resulting in the production of an APPsβ/FLAG fusion protein and deletion of Aβ and intracellular sequences (Fig. 1A and Fig. S1). Similar to the APPsα ki mice (23), homozygous APPsβ ki (ki/ki) mice are phenotypically normal with respect to overall growth and general brain morphology (Fig. S2). Quantitative real-time PCR (qRT-PCR) analysis showed that compared with their littermate WT controls, there was an ∼50% reduction of APP transcript in the ki/ki brain (Fig. 1B), which might result from insufficient poly(A) addition by the human growth hormone poly(A) sequence introduced in the ki construct. This reduction correlated with a similar reduction of total APPsβ protein levels (Fig. 1C, 22C11 panel, and Fig. 1D, quantified). Blotting with an anti-FLAG antibody confirmed the expression of the FLAG-tagged fusion protein at ∼100 kDa only from the ki mice but not from WT littermates (Fig. 1C, FLAG). An anti-APP C-terminal (APPc) antibody showed positive detection only in WT but not ki/ki samples (Fig. 1C, APPc), validating the lack of APP C-terminal sequences in the APPsβ ki mice. Even though the total APP expressed in ki/ki mice was reduced, quantitative analysis of PBS-extractable soluble APP revealed no significant differences between the ki/ki mice and their WT littermates (Fig. 1 E and F), suggesting that the APPsβ produced in the ki mice was more efficiently secreted. Quantitative immunoblotting of spinal cord total protein lysates and soluble fractions yielded similar results (Fig. S3).
Fig. 1.
Generation and biochemical characterization of APPsβ ki mice. (A) Schematic representation of WT and APPsβ ki alleles. Cleavage sites for α-, β-, and γ-secretase are indicated by α, β, and γ, respectively. In the ki allele, a FLAG sequence followed by a stop codon was inserted immediately downstream of the β-secretase site. TM, transmembrane region. (B) qRT-PCR of APP mRNA in 2-mo-old homozygous APPsβ ki/ki and APP KO (−/−) mouse brains relative to WT controls (+/+), showing an ∼50% reduction of APP mRNA in ki/ki samples and a negligible amount in APP−/− samples as compared with the WT controls. (C) Western blot analysis of APP expression in total brain lysate from 2-mo-old ki/ki mice and their WT (+/+) littermates using the 22C11, APPc, and anti-FLAG antibodies. A γ-tubulin blot was used as a loading control. (D) Quantification of the relative ratio of 22C11/γ-tubulin blots. (E) Western blot analysis of PBS-extractable soluble APP in +/+ and ki/ki brains using the 22C11 antibody. A γ-tubulin blot was used as a protein loading control. (F) Quantification of the Western blots in E. (G) Representative Western blots of APP in TCL and CM from cultured WT (+/+) and homozygous APPsβ (ki/ki) primary neurons using the 22C11 antibody. A γ-tubulin blot was used as a loading control. (H) Quantification of the ratio of APP/tubulin in TCL of +/+ and ki/ki cultures. (I) Quantification of the ratio of APP/tubulin in CM of +/+ and ki/ki cultures. Note the absence of any cleavage products in the TCL or CM. The ratio of APP/tubulin in +/+ samples was normalized to 1 in all quantifications. **P < 0.01; ***P < 0.001. N.S., nonsignificant (P > 0.05, t test).
To investigate the processing and secretion of APPsβ, we prepared neuronal cultures from postnatal day 0 (P0) ki/ki pups and littermate WT (+/+) controls. Total cell lysates (TCLs) and conditioned medium (CM) were collected at 14 d in vitro (DIV), and APP protein levels were assayed by immunoblotting (Fig. 1G). Consistent with the observations made with brain tissue, cell-associated APP was significantly reduced in the ki/ki neurons (Fig. 1H) but secreted APP was comparable between the two genotypes (Fig. 1I). This result agrees with the finding that deletion of the carboxyl sequences of APP promotes its secretion (25). Of note, we consistently detected two bands in the CM of ki/ki samples (Fig. 1G). Although the exact nature of the two bands is not clear, they appear to be a unique property of the cultured neurons because the extra band was not detected in brain samples or cultured cell lysates.
Nikolaev et al. (3) reported that trophic factor deprivation triggers the release of APPsβ, which undergoes further cleavage by an unknown protease to generate an ∼35-kDa N-APP. The production of only APPsβ protein in our ki animals provided a unique system to investigate the stability and possible cleavage of APPsβ in the absence of APPsα. Western blotting of WT and ki/ki samples using the 22C11 N-APP antibody revealed that APPsβ existed as a nonproteolyzed protein in both the cell lysates and CM (Fig. 1G). The intact nature of the APPsβ protein was further evidenced by analyzing CM taken from WT, APPsβ heterozygous (ki/+), or APPsβ homozygous (ki/ki) neurons using the C-terminal anti-FLAG antibody at various cultured stages (Fig. S4A) and when expressed at exceedingly high concentrations (Fig. S4B). Finally, analysis of APPsβ development in the ki brains during embryonic day (E) 14.5 and E16.5 and P0 and P14 also failed to detect any cleavage products using both the N-terminal 22C11 (Fig. S4C) and C-terminal anti-FLAG antibodies (Fig. S4D). These results strongly argue that the secreted APPsβ protein is stable in vivo and does not undergo further proteolytic cleavage under regular cell culture conditions in vitro.
Expression of APPsβ on an APLP2 Null Background Leads to Early Postnatal Lethality and Severe Neuromuscular Synapse Defects.
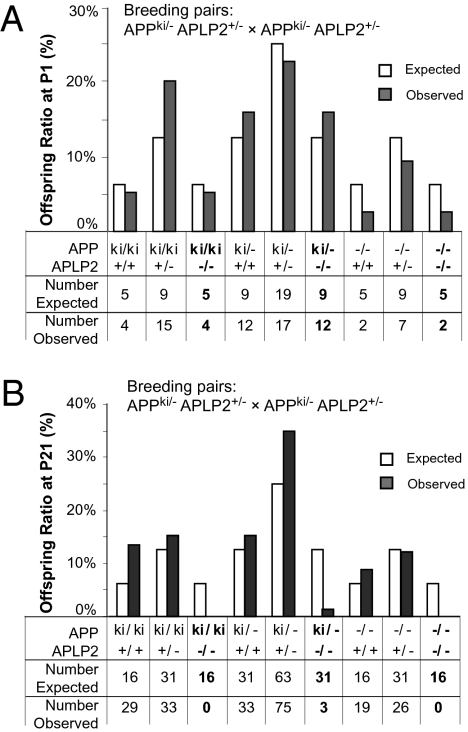
Hornsten et al. (24) reported that neuronal expression of a soluble APL-1 extracellular protein is sufficient to rescue the lethality of C. elegans mutant deleting apl-1. To assess whether APPsβ is able to rescue the lethal phenotype of the APP/APLP2 dKO mice, we intercrossed double-heterozygous mice harboring one allele each of the APPsβ and APLP2 null mutations (APPki/−APLP2+/−). We then determined the genotypes of the offspring at P1 and P21 and compared the observed and expected numbers (Fig. 2). Genotyping of P1 pups revealed a close to Mendelian distribution of all genotypes, indicating no embryonic lethality as expected (χ2 = 10.65, P > 0.1). However, genotyping of 218 offspring from the same cross at weaning age (P21) identified very few surviving APPki/kiAPLP2−/−, APPki/−APLP2−/−, or APP−/−APLP2−/− mice, the number of which significantly deviated from the predicted Mendelian ratio (χ2 = 79.6, P < 0.001). These results demonstrate that contrary to the C. elegans finding, expression and secretion of APPsβ cannot rescue the postnatal lethality of the APP/APLP2 double-deficient mice.
Fig. 2.
Survival analysis of APPsβ ki mice on APLP2 null background. (A) Analysis of genotypes of 75 offspring collected at P1 derived from crosses of APPki/−APLP2+/− male and female mice. All genotypes were recovered at close to a Mendelian ratio (8 df, χ2 = 10.65, P > 0.1). (B) Analysis of genotypes of 218 offspring collected at P21 derived from the same breeding as in A. The number of APPki/kiAPLP2−/−, APPki/−APLP2−/−, or APP−/−APLP2−/− animals observed was much lower than expected (highlighted in bold) (8 df, χ2 = 79.6, P < 0.001).
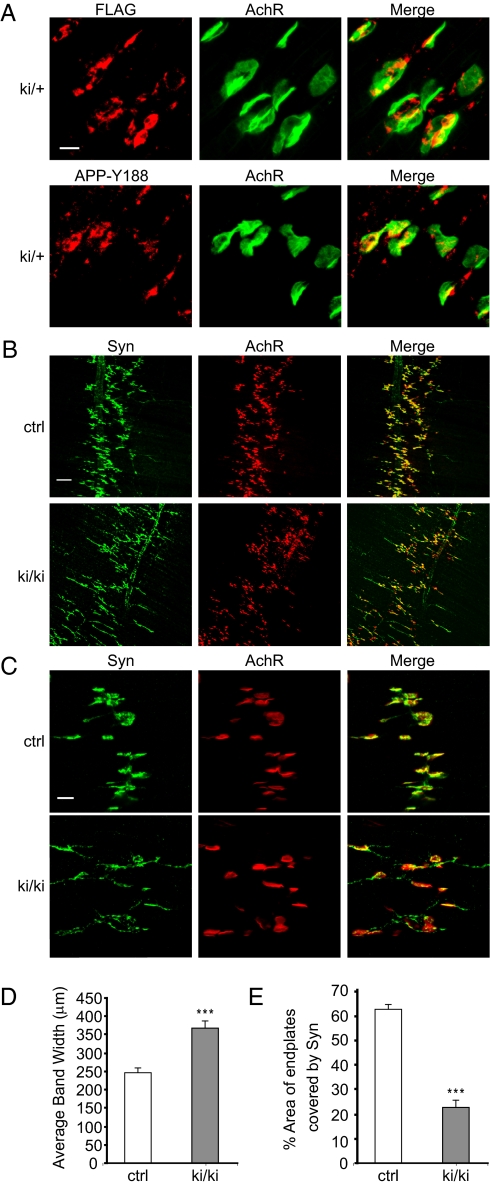
We previously reported that APP is targeted to the synaptic sites of the NMJ (26). We performed localization analysis of APPsβ using the anti-FLAG antibody in heterozygous APPsβ ki muscle preparations. Interestingly, the staining pattern was indistinguishable from that of WT full-length APP recognized by the C-terminal APP antibody Y188 (Fig. 3A), suggesting that the APPsβ can be targeted to the synaptic terminals without the C-terminal sequences, likely through a mechanism independent of sorting signals (27). Despite the apparent synaptic expression of APPsβ, crossing the APPsβ ki with APLP2 KO mice and staining of neuromuscular synapses at P0 showed that compared with APP+/+APLP2−/− littermate controls, APPki/kiAPLP2−/− mutants exhibited expanded neuromuscular synapses (Fig. 3 B and D, quantified in the latter), with significant localization of synaptic vesicle proteins in extrasynaptic compartments and a correspondingly reduced apposition of presynaptic markers with postsynaptic receptors (Fig. 3 C and E, quantified in the latter). The degree of the defects was similar to that of the dKO animals characterized previously (21, 26). These results demonstrate that APPsβ is inactive in APP-mediated survival and neuromuscular synapse function.
Fig. 3.
Analysis of neuromuscular synapse phenotypes of APPsβ ki mice. (A) Immunofluorescence staining of P0 sternomastoid muscle sections of heterozygous APPsβ ki animals (ki/+) using an anti-FLAG antibody and the anti-APP antibody Y188. Staining with α-bungarotoxin was used to mark the postsynaptic AchRs. (Merge) Overlay of APP and AchR images. (B) Whole-mount staining of P0 diaphragm muscles of APPki/kiAPLP2−/− mutants (ki/ki) and littermate APP+/+APLP2−/− controls (ctrl) with an anti-synaptophysin (Syn) antibody and α-bungarotoxin (AchR) showing diffused pre- and postsynaptic distribution in the ki/ki mutant. (Merge) Overlay of Syn and AchR images. (C) Higher magnification images of synapse structures showing axonal staining of Syn and poorly covered end plates by Syn in the ki/ki mutant. (D) Quantification of the average bandwidth of AchR-positive end plates. (E) Quantification of the percentage of AchR-positive end plates covered by Syn (average ± SEM of 20 end plates per genotype). ***P < 0.001 (Student's t test). (Scale bar: A and C, 20 μM; B, 100 μM.)
Transcriptional Profiling of Neuronal APP/APLP2 Double-Conditional KO Mice.
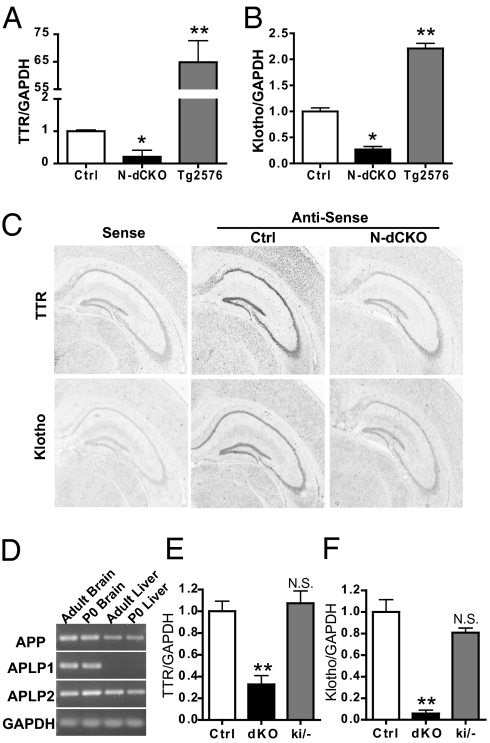
APP has been implicated in transcriptional regulation through its intracellular domain. However, our microarray analysis using adult APP null hippocampal samples or P0 APP/APLP2 dKO brains failed to detect appreciable gene expression changes. We reasoned that this might be attributable to the compensatory mechanisms of APLP2 in the case of APP single-KO brains and the mixed cell types collected at P0 in APP/APLP2 dKO brains, which may dilute any tissue-specific differences. We created an APP floxed allele and observed that in contrast to the APP/APLP2 dKO mice, neuronal-specific APP/APLP2 double-conditional KO (N-dCKO) mice are viable. This allowed us to bypass the lethality or redundancy issues (26). Indeed, transcriptional profiling of hippocampi dissected from 2-mo-old N-dCKO mice and littermate APLP2 null controls uncovered ∼30 genes that showed significant differences between N-dCKO mutants and the controls, with the vast majority down-regulated in N-dCKO samples (Table S1). We compared our list with published microarray data using APP-overexpressing animals before amyloid deposition (28, 29) and chose to focus on TTR and Klotho because their expression was down-regulated in N-dCKO samples and up-regulated in APP transgenic brains. The changes in TTR and Klotho expression as a function of APP gene dosage were confirmed by qRT-PCR analysis (Fig. 4 A and B). Reduced TTR and Klotho transcripts in the hippocampus were also confirmed by in situ hybridization (Fig. 4C). In line with the negative microarray results obtained with newborn samples, qRT-PCR of TTR and Klotho in P0 brains showed no significant differences between the APLP2 null control and the dKO mutant (Fig. S5). Consistent with the compensatory role of APLP2, expression of TTR and Klotho was comparable between the APP single-KO and WT hippocampal samples (Fig. S5).
Fig. 4.
Analysis of TTR and Klotho expression. Relative qRT-PCR analysis of TTR (A) and Klotho (B) mRNA levels from the hippocampi of 2-mo-old control (Ctrl), N-dCKO, and Tg2576 APP transgenic mice (n = 3). (C) Representative in situ hybridization images of TTR and Klotho from littermate APLP2−/− control (Ctrl) and N-dCKO brain sections (sense, hybridization with a sense probe as a negative control; antisense, hybridization using corresponding antisense probes). (D) RT-PCR analysis of APP, APLP1, and APLP2 expression in adult and P0 brain and liver. GAPDH was used as an amplification control. Relative qRT-PCR analysis of TTR (E) and Klotho (F) mRNA levels in P0 livers from control APLP2 null (Ctrl), APP, and APLP2 dKO and APPsβ ki mice on APLP2 null background (ki/−). Data are the mean ± SEM of two independent experiments, each with three samples per genotype. *P < 0.05; **P < 0.01. (N.S., nonsignificant, Student's t test).
APP has been proposed to regulate gene transcription by first recruiting Fe65 to the membrane before γ-secretase cleavage, activating Fe65 by an unknown mechanism at the membrane and then releasing Fe65, together with the AICD, by γ-secretase cleavage, thereby allowing Fe65 to enter the nucleus and to interact with the chromatin remodeling factor Tip60 (4, 5). As such, the most straightforward explanation for the altered TTR and Klotho expression in the APP/APLP2 double-mutant mice would be that TTR and Klotho are direct targets of the Fe65-mediated signaling pathway. We tested this possibility by cotransfecting an APPC99 expression vector with or without Fe65 and a luciferase reporter construct driven by the 3-kb TTR promoter (30) but failed to detect significant induction of luciferase activities (Fig. S6). These results indicate that other APP regions, such as the APP extracellular motifs, might be involved in modulating TTR and Klotho expression, a hypothesis that could presumably be tested using the APPsβ ki mice. However, the early postnatal lethality of the APPki/−APLP2−/− mice limited our analysis to the newborn stage, and analysis of APP/APLP2 dKO brains at P0 failed to establish a reduction of TTR or Klotho (Fig. S5). We reasoned that this could be attributed to low levels of TTR and Klotho expression and/or the compensatory activity of APLP1 in the P0 brain. We chose to examine the expression of these targets in the P0 liver because (i) TTR is known to be highly expressed in the liver and (ii) APLP1 is expected to be a neuronal protein not expressed in peripheral tissues. Indeed, the absence of APLP1 in the newborn and adult liver tissues was confirmed by qRT-PCR (Fig. 4D). Therefore, at P0, there is no expression of any APP-related proteins in the dKO mouse liver, and the only APP family of proteins present in the APPki/−APLP2−/− sample is soluble APPsβ. We were able to identify a significant decrease of both TTR (Fig. 4E) and Klotho (Fig. 4F) mRNA levels in the P0 dKO liver. Importantly, this reduction was not seen in APPki/−APLP2−/− samples (Fig. 4 E and F, ki/−), demonstrating that expression of APPsβ is sufficient to support the TTR and Klotho expression. As such, the regulation of TTR and Klotho is mediated by APPsβ and likely through a receptor distinct from the APP family proteins.
Discussion
We report here that (i) APPsβ is highly stable in the central nervous system in vivo and under regular culture conditions in vitro, (ii) expression of APPsβ is not sufficient to rescue the early postnatal lethality and neuromuscular synapse defects of the APP/APLP2 null mice, and (iii) TTR and Klotho are previously undescribed targets of APP whose transcripts are maintained by APPsβ. Our findings support the notion that secreted APPsβ can elicit an intercellular signal independent of full-length APP but that full-length APP is required for neuromuscular synapse assembly and postnatal survival.
Loss-of-function studies established an essential and redundant role of the APP family of proteins in postnatal survival (20, 31). This essential activity is shared in lower organisms, because C. elegans deficient in apl-1 also dies at the L1 stage (24). Nevertheless, the apl-1 null lethal phenotype can be rescued by neuronal expression of the APL-1 extracellular domain, whereas expression of APPsβ is unable to rescue the lethality of APP/APLP2 null mice. Although it remains possible that the lethality of APP/APLP2 null mice could be rescued by APPsα, we believe this is unlikely because we have created another strain of APP ki allele expressing a membrane-anchored APP lacking the last 39 amino acids. Although both APPsα and APPsβ are readily detectable in these mice, these animals display a similar lethality when expressed on an APLP2 KO background (32). The reason for the distinct sequence requirement for C. elegans and mouse survival is not known, but it is worth pointing out that the lethality of the apl-1–deficient worm is attributable to a molting defect that is not relevant for mammals.
Our finding that APPsβ fails to rescue the NMJ defects of the APP/APLP2 null mice is consistent with our conditional KO studies indicating that full-length APP is necessary for its synaptogenic activity (26). The apparently normal synaptic localization of APPsβ may not be in agreement with various studies showing that kinesin-dependent axonal trafficking of APP requires its intracellular sequences (33), but it is consistent with a recent report that fast anterograde transport of APP does not require the AICD or any known sorting signal (27). It is important to note that the immunostaining method used here does not offer sufficient spatial resolution to define the localization of the APPsβ protein precisely and does not address whether the APPsβ detected is axonally transported. As such, defective axonal transport of APPsβ cannot be formally excluded.
A recent publication by Nikolaev et al. (3) reported that APPsβ, via a cleaved N-terminal derivative, binds to the DR6 receptor and mediates axonal pruning and neuronal cell death under conditions of trophic withdrawal. Our results that APPsβ is highly stable and that APPsβ fails to rescue the nerve-sprouting phenotype of the APP/APLP2 null NMJ are at odds with these findings. Although we cannot exclude the possibility that APPsβ undergoes further cleavage under specific circumstances, such as those used by Nikolaev et al. (3), our data suggest that APPsβ exists as a stable protein rather than cleaved fragments and that the neuromuscular synapse defects present in APP/APLP2 null mice are not caused by the lack of APPsβ or, by extension, a defective APPsβ/DR6 pathway.
The down-regulation of TTR and Klotho in APP/APLP2 loss-of-function mutants and their up-regulation in APP-overexpressing mice provide strong support for the notion that they are the direct targets of APP. Because expression of APPsβ is sufficient to restore the expression of TTR and Klotho in the liver of APPki/−APLP2−/− mice in which no APP or any of its family members is present, regulation of TTR and Klotho expression must be mediated by APPsβ independent of full-length APP. Although not conflicting with the putative transcriptional activity of APP (4, 5), our results provide an alternative mechanism whereby the secreted APP, via binding to an unknown receptor(s), activates downstream target genes, including TTR and Klotho. It should be noted that the link of Fe65 to chromatin remodeling instead of transcription suggests that the AICD probably does not act on specific genes but modulates the overall transcriptional state of a cell (17).
The functional role of APP-mediated TTR and Klotho expression remains to be established. Because expression of APPsβ cannot complement the developmental activities of APP, TTR and Klotho do not appear to mediate essential functions of APP. With regard to the candidate receptors, secreted APP has been shown (among other receptors) to bind to low density lipoprotein receptor related protein (34) and class A scavenger receptor (35). Caille et al. (36) indicated the presence of binding sites for APPs in EGF-responsive neural stem cells of the adult rodent brain. As such, APPs could presumably stimulate downstream signaling through EGF receptor- or LRP-mediated pathways. Alternatively, APP or APPs has been shown to interact with integrin β1 (37), netrin-1 (38), and reelin (39). These protein-protein interactions could, in principle, trigger intracellular events leading to TTR and Klotho expression. The ability to restore liver TTR and Klotho by APPsβ makes it a legitimate assumption that the receptor is expressed in the liver. Although DR6 cannot be formally excluded as a potential APPsβ receptor, the absence of N-APP in our system argues against a functional role of this pathway. Nevertheless, identification of the receptor(s) and intracellular pathways leading to TTR and Klotho regulation awaits further investigation.
Because Aβ is an integral component of the highly regulated APP proteolytic cleavage cascade, misregulation of APP processing and the APP-dependent signaling pathway as well as Aβ accumulation may contribute to neuronal dysfunction and Alzheimer's disease (AD) pathogenesis. In light of the well-documented role of TTR in Aβ sequestration and amyloid suppression (40, 41) and of Klotho in various aging processes, including oxidative stress and calcium homeostasis (42–44), the identification of these proteins as the APP targets has direct implications for AD pathogenesis. In mice, TTR haploinsufficiency leads to enhanced amyloid pathology in an APP/presinilin mouse model (41), and TTR protects Aβ-induced toxicity and behavioral impairment (45). TTR levels are decreased in the cerebrospinal fluid of patients with AD, suggesting a lack of a protective mechanism in the diseased brain (46, 47). We propose a model whereby extracellular APP processing produces APP ectodomain derivatives, which promote TTR and Klotho expression and protect against Aβ neurotoxicity during aging. The disruption of this homeostasis, which can be brought about by reduced APPs and/or elevated Aβ, both associated in individuals with AD (48), may contribute to AD pathogenesis during aging.
Methods
Antibodies and Reagents.
22C11 and Y188 were monoclonal antibodies recognizing the amino (amino acids 66–81) and carboxyl (containing YENPTY) sequences of APP and were obtained from Millipore and Epitomics, respectively. The polyclonal APPc was described previously (49). Anti-FLAG (rabbit polyclonal) and anti-V5 (mouse monoclonal) antibodies were purchased from Sigma and Abcam, respectively, and the antisynaptophysin polyclonal antibody was from DAKO. The α-bungarotoxin was from Molecular Probes.
Animals.
Neuronal APP and APLP2 N-dCKO mice were derived by crossing APP floxed mice with transgenic mice expressing the Cre-recombinase under the neuronal rat nestin promoter, followed by breeding with APLP2 null animals (26, 50). The detailed method for constructing the APPsβ ki mice can be found in SI Text and Fig. S1. All the strains used have been backcrossed onto C57BL/6J background for at least six generations.
qRT-PCR.
Total RNA was isolated from brain or liver. Reverse transcription was performed using the SuperScript III first-strand synthesis system for the RT-PCR kit (Invitrogen), and the reaction mix was subjected to qRT-PCR using the ABI PRISM Sequence Detection System 7000 (Applied Biosystems). Primers were designed with Primer Express Version 2.0 software (Applied Biosystems) using sequence data from the National Center for Biotechnology Information. GAPDH primers were used as an internal control for each specific gene amplification. The relative levels of expression were quantified and analyzed using ABI PRISM Sequence Detection System 7000 software. The real-time value for each sample was averaged and compared using the comparative cycle threshold (CT) method. The relative amount of target RNA was calculated relative to the expression of endogenous reference and relative to a calibrator, which was the mean CT of control samples.
In Situ Hybridization.
Coronal serial sections (20 μm in thickness) of heads of 2-mo-old APLP2−/− control and N-dCKO mice were cut with a cryostat and placed with adjacent sections on separate slides. After paraformaldehyde fixation and acetylation, the slides were assembled into flow-through hybridization chambers and placed in a Tecan Genesis 200 liquid-handling robot (Mannedorf). Templates for synthesis of digoxigenin-labeled riboprobes for TTR and Klotho were full-length TTR cDNA and the N-terminal 810 bp of Klotho cDNA, respectively, in pCR-Blunt II-TOPO vectors (Invitrogen). Antisense and sense probes were transcribed with T7 and Sp6 polymerase, respectively, from linearized vector. Hybridized probes were detected by catalyzed reporter deposition using biotinylated tyramide; this was followed by colorimetric detection of biotin with avidin coupled to alkaline phosphatase. Hybridization with sense control probes did not yield signals above background.
Cell Culture, Western Blotting, and Immunostaining.
The primary hippocampal culture was prepared as described (26). CM and TCL (in PBS with complete protease inhibitor mixture) were collected at 14 DIV. For the time-course experiment, 10% of the total volume of neuronal culture CM was taken out at times indicated for Western blotting analysis and replenished with an equal volume of fresh serum-free medium. Western blot analysis and immunofluorescence staining were performed and analyzed as previously described (26).
Statistical Analysis.
Genotyping analysis of the offspring from APPki/−APLP2+/− male and female intercrosses was performed using χ2 analysis. The Student's t test was used for all other analyses (*P < 0.05; **P < 0.01; ***P < 0.001). Data were presented as the average ± SEM.
Supplementary Material
Acknowledgments
We thank R. Atkinson of the confocal core, C. Thaller and A. Liang of the RNA in situ core, and the Baylor College of Medicine Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Center (Grant P30HD024064) for resources and support. We thank X. Chen and N. Aithmitti for expert technical assistance and members of the Zheng laboratory for stimulating discussions. This work was supported by National Institutes of Health Grants AG032051 and AG033467 (to H.Z.) and Grant MH52804 (to T.C.S.) and by American Health and Assistance Foundation Grant A2008-052 (to H.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012568107/-/DCSupplemental.
References
- 1.Zheng H, Koo EH. The amyloid precursor protein: Beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaev A, McLaughlin T, O'Leary DDM, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Cao X, Südhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 5.Cao X, Südhof TC. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J Biol Chem. 2004;279:24601–24611. doi: 10.1074/jbc.M402248200. [DOI] [PubMed] [Google Scholar]
- 6.Baek SH, et al. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, et al. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3beta expression. FASEB J. 2003;17:1951–1953. doi: 10.1096/fj.03-0106fje. [DOI] [PubMed] [Google Scholar]
- 8.Pardossi-Piquard R, et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YW, et al. Presenilin/gamma-secretase-dependent processing of beta-amyloid precursor protein regulates EGF receptor expression. Proc Natl Acad Sci USA. 2007;104:10613–10618. doi: 10.1073/pnas.0703903104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Rotz RC, et al. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci. 2004;117:4435–4448. doi: 10.1242/jcs.01323. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Cool BH, Martin GM, Hu Q. A dominant role for FE65 (APBB1) in nuclear signaling. J Biol Chem. 2006;281:4207–4214. doi: 10.1074/jbc.M508445200. [DOI] [PubMed] [Google Scholar]
- 13.Hebert SS, et al. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen AC, Selkoe DJ. Response to: Pardossi-Piquard et al., “Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP.” Neuron 46:541–554. Neuron. 2007;53:479–483. doi: 10.1016/j.neuron.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Repetto E, Yoon IS, Zheng H, Kang DE. Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J Biol Chem. 2007;282:31504–31516. doi: 10.1074/jbc.M704273200. [DOI] [PubMed] [Google Scholar]
- 16.Tamboli IY, et al. Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis. J Neurosci. 2008;28:12097–12106. doi: 10.1523/JNEUROSCI.2635-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giliberto L, et al. Evidence that the Amyloid beta Precursor Protein-intracellular domain lowers the stress threshold of neurons and has a “regulated” transcriptional role. Mol Neurodegener. 2008;3:12. doi: 10.1186/1750-1326-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H, et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 19.Dawson GR, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 20.von Koch CS, et al. Generation of APLP2 KO mice and early postnatal lethality in APLP2/APP double KO mice. Neurobiol Aging. 1997;18:661–669. doi: 10.1016/s0197-4580(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King GD, Scott Turner R. Adaptor protein interactions: Modulators of amyloid precursor protein metabolism and Alzheimer's disease risk? Exp Neurol. 2004;185:208–219. doi: 10.1016/j.expneurol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Ring S, et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornsten A, et al. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA. 2007;104:1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, et al. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Back S, et al. beta-amyloid precursor protein can be transported independent of any sorting signal to the axonal and dendritic compartment. J Neurosci Res. 2007;85:2580–2590. doi: 10.1002/jnr.21239. [DOI] [PubMed] [Google Scholar]
- 28.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu ZL, et al. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol Aging. 2006;27:377–386. doi: 10.1016/j.neurobiolaging.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Liao L, et al. Liver-specific overexpression of the insulin-like growth factor-I enhances somatic growth and partially prevents the effects of growth hormone deficiency. Endocrinology. 2006;147:3877–3888. doi: 10.1210/en.2005-1537. [DOI] [PubMed] [Google Scholar]
- 31.Herms J, et al. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, et al. Genetic dissection of the amyloid precursor protein in developmental function and amyloid pathogenesis. J Biol Chem. 2010 doi: 10.1074/jbc.M110.137729. 10.1074/jbc.M110.137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisodia SS. Biomedicine. A cargo receptor mystery APParently solved? Science. 2002;295:805–807. doi: 10.1126/science.1069661. [DOI] [PubMed] [Google Scholar]
- 34.Kounnas MZ, et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 35.Santiago-García J, Mas-Oliva J, Innerarity TL, Pitas RE. Secreted forms of the amyloid-beta precursor protein are ligands for the class A scavenger receptor. J Biol Chem. 2001;276:30655–30661. doi: 10.1074/jbc.M102879200. [DOI] [PubMed] [Google Scholar]
- 36.Caille I, et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 37.Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Develop. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lourenço FC, et al. Netrin-1 interacts with amyloid precursor protein and regulates amyloid-beta production. Cell Death Differ. 2009;16:655–663. doi: 10.1038/cdd.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoe HS, et al. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci. 2009;29:7459–7473. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarzman AL, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci USA. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SH, et al. Accelerated Abeta deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin) J Neurosci. 2007;27:7006–7010. doi: 10.1523/JNEUROSCI.1919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 43.Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imura A, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 45.Buxbaum JN, et al. Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Abeta toxicity. Proc Natl Acad Sci USA. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: Aging and late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63:506–508. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merched A, et al. Apolipoprotein E, transthyretin and actin in the CSF of Alzheimer's patients: Relation with the senile plaques and cytoskeleton biochemistry. FEBS Lett. 1998;425:225–228. doi: 10.1016/s0014-5793(98)00234-8. [DOI] [PubMed] [Google Scholar]
- 48.Palmert MR, et al. Soluble derivatives of the beta amyloid protein precursor in cerebrospinal fluid: Alterations in normal aging and in Alzheimer's disease. Neurology. 1990;40:1028–1034. doi: 10.1212/wnl.40.7.1028. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Yang L, Wang Z, Zheng H. Amyloid precursor protein mediates presynaptic localization and activity of the high-affinity choline transporter. Proc Natl Acad Sci USA. 2007;104:14140–14145. doi: 10.1073/pnas.0704070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.