Abstract
Ecologists have long observed that consumers can maintain species diversity in communities of their prey. Many theories of how consumers mediate diversity invoke a tradeoff between species’ competitive ability and their ability to withstand predation. Under this constraint, the best competitors are also most susceptible to consumers, preventing them from excluding other species. However, empirical evidence for competition–defense tradeoffs is limited and, as such, the mechanisms by which consumers regulate diversity remain uncertain. We performed a meta-analysis of 36 studies to evaluate the prevalence of the competition–defense tradeoff and its role in maintaining diversity in plant communities. We quantified species’ responses to experimental resource addition and consumer removal as estimates of competitive ability and resistance to consumers, respectively. With this analysis, we found mixed empirical evidence for a competition–defense tradeoff; in fact, competitive ability tended to be weakly positively correlated with defense overall. However, when present, negative relationships between competitive ability and defense influenced species diversity in the manner predicted by theory. In the minority of communities for which a tradeoff was detected, species evenness was higher, and resource addition and consumer removal reduced diversity. Our analysis reframes the commonly held notion that consumers structure plant communities through a competition–defense tradeoff. Such a tradeoff can maintain diversity when present, but negative correlations between competitive ability and defense were less common than is often assumed. In this respect, this study supports an emerging theoretical paradigm in which predation interacts with competition to both enhance and reduce species diversity.
Keywords: meta-analysis, resource limitation, predation, species diversity
Identifying processes that maintain species diversity in the face of competitive exclusion is a key goal of ecology (1). Because consumers can alter the outcome of competition between their prey, consumer-based mechanisms are commonly invoked to explain species coexistence (2–4). Many empirical (5–7) and theoretical studies (8–10) have suggested that consumers maintain species diversity when predation differentially harms superior competitors. For example, Lubchenco (3) showed that snail herbivory increased algal diversity in tide pools only when preferred prey were also the competitive dominant. Similar requirements for consumers to maintain diversity of their prey have been formalized in mathematical models: when competing species share both resources and consumers, coexistence is possible only if the prey species that are superior competitors for resources are also less resistant to predation (9, 10).
However, a large gap has developed between the empirical evidence supporting this theoretical tradeoff and its application to explain how consumers regulate real communities. For the many studies that have invoked a tradeoff between competitive ability and defense against consumers, the mechanism is more often assumed than directly demonstrated (11). Few studies have evaluated the strength of this tradeoff across species (12–14), largely due to the challenge of quantifying species’ abilities to compete for resources and to defend themselves against consumers. Instead, many studies that have been put forth as support for the tradeoff actually focus on trait differences between individuals of a single species (ref. 11 and references therein). Such studies offer only limited insight into the operation of the tradeoff at the community level (i.e., across species). Therefore, though it is clear that consumers have some effect on species diversity, it remains unclear whether a competition–defense tradeoff is a widespread mechanism underlying consumer effects.
In this paper, we describe a 36-study meta-analysis conducted to quantify the relationship between competitive ability and defense and to evaluate its role in maintaining species diversity in plant communities. Plants use several types of defenses to protect themselves against consumers. However, we focus solely on the resistance component of defense, because past theoretical work has made clear that only a tradeoff between competitive ability and resistance promotes coexistence (9, 15) (SI Text). We adopt an approach that allows us to estimate competitive ability and defense across numerous studies. Specifically, we infer competitive ability and resistance to consumers by quantifying species’ responses to experimental resource addition and consumer removal, respectively (Figs. 1 and 2). Because numerous studies have simultaneously manipulated these factors, this approach dramatically expands the pool of studies available for examining tradeoffs. Furthermore, because it was not the original intent of such studies to assess the competition–defense tradeoff, these studies provide what can be considered unbiased estimates of the relationship between competitive ability and resistance to consumers.
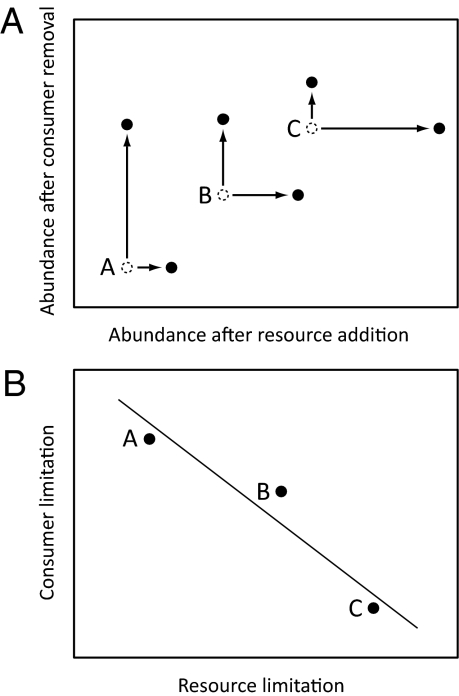
Fig. 1.
A hypothetical community used to demonstrate the study approach. (A) Species’ abundances were measured following either resource addition or consumer removal (solid symbols). Open symbols represent their premanipulation (control) abundance. Arrows indicate the change between control and experimental treatments, or the strength of resource and consumer limitation for each species. Species A is a strong resource competitor but very consumer-limited; species C is resistant to consumers but resource-limited; and species B is intermediate in both respects. (B) The competition–defense correlation quantifies the relationship between resource and consumer limitation [the size of the horizontal and vertical arrows, respectively, in A]. A negative correlation indicates the existence of a competition–defense tradeoff.
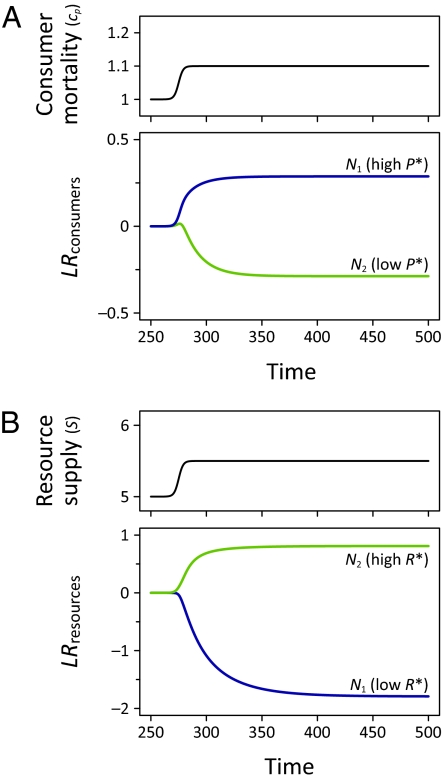
Fig. 2.
Responses of two species with a competition–defense tradeoff to simulated perturbations in resource supply and consumer mortality. The responses in the large panels are species’ log response ratios relative to their preperturbation densities (defined at t = 250). Perturbations are included for reference in the small panels. (A) The species more limited by predation (species 1) benefits more from the decrease in consumer pressure than the resistant species (species 2). (B) The poor resource competitor (species 2) benefits more from resource addition than the strong resource competitor (species 1). See SI Text for a full description of the model.
Our approach relies on the expectation that the species most sensitive to the limiting resources, the poorest competitors, benefit most from resource addition (16). Likewise, those most limited by predation benefit most from consumer removal. Therefore, if a competition–defense tradeoff exists, the species that respond most strongly to resource addition will respond least strongly to consumer removal, and vice versa (Fig. 1). These expectations reflect a common theoretical view of how populations respond when the intensity of resource limitation or consumer pressure changes (8–10, 16). Models incorporating competition–defense tradeoffs have shown that when resource availability and consumer pressure are low, communities are dominated by the species that are superior at obtaining resources but vulnerable to consumers (9, 10). As resource availability and consumers increase, dominance shifts to resistant species that are poor at resource exploitation. If the resource and consumer manipulations used in our meta-analysis studies are viewed as discrete points along the continuous gradients used in such models, then increasing resource availability would benefit poor competitors, and reducing consumer pressure would benefit species vulnerable to predation. Indeed, there is strong empirical support that species’ responses to the experimental manipulations conform to our expectations about resource and consumer limitation (17–19). For example, competitive ability of 27 grassland species was negatively correlated with their increase in abundance along a fertilization gradient (18).
We have tested our expectations using simulations of one of the foremost models underlying the competition–defense tradeoff (see SI Text for full details). The model (9) is based on the common R* framework of competitive ability, where R* is the level to which a species depletes resources when grown in monoculture (16). Species strongly limited by resources have high R*s. Likewise, P* is the consumer density that a prey species can support in monoculture, with large values reflecting strong consumer limitation (9). We imposed perturbations in resource supply and consumer mortality on two competing species with a tradeoff between resource and consumer limitation (Figs. S1 and S2). The change in both species’ densities relative to their preperturbation densities supports our expectations (Fig. 2). The species that is more limited by consumers (species 1) benefited more from increasing consumer mortality (Fig. 2A), whereas the species more limited by resource availability (species 2) benefited more from resource addition (Fig. 2B). In SI Text, we also show that this result is robust to our specific parameter selection (Fig. S3).
To assess whether similar trends are evident in empirical studies, we quantify the strength of the competition–defense tradeoff as the correlation between species’ responses to resource addition and consumer removal (the competition–defense correlation; Fig. 1B). Negative correlations indicate the existence of a tradeoff. Because the influence of resources and consumers on prey diversity may depend on factors that varied across studies (20), we examine competition–defense correlations of studies partitioned by the characteristics of the species and systems involved (Table S1). Finally, we test the theoretical prediction that the tradeoff maintains diversity (9, 10). Specifically, we use our dataset to test the following hypotheses: (i) diversity of plant communities increases with the strength of the competition–defense tradeoff; and (ii) in communities for which a tradeoff is observed, removal of competition or predation reduces plant species diversity. As we will show, evidence for a widespread competition–defense tradeoff is equivocal. Even so, the relationship between competitive ability and defense against herbivores still yields valuable insight into the processes that regulate diversity within plant communities.
Results
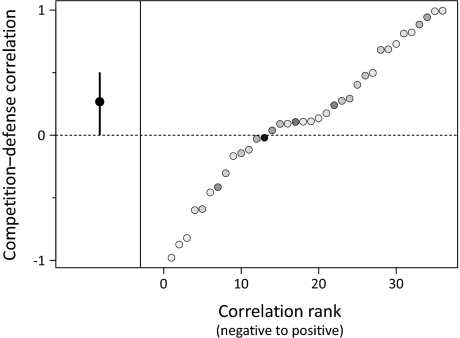
In contrast to theoretical predictions, the correlation between plant species’ response to resource addition and their response to consumer removal was positive when averaged across all studies. Though the overall correlation was significantly positive (P = 0.046), it was relatively small in magnitude and resulted from the fact that only slightly more studies showed positive correlations than had the theoretically expected negative correlation (Fig. 3; of the 36 studies, 13 exhibited a negative correlation between species’ responses to resource addition and to consumer removal, as is predicted by a tradeoff). Combined correlations were qualitatively unaffected by whether studies were weighted or not weighted to account for certainty in measurements, and they were not affected by the choice of the time point that was analyzed for studies with time-series data available (Methods). Taken as a whole, these findings suggest that a competition–defense tradeoff is far from a ubiquitous feature of plant communities. Rather, our analysis suggests that plant species that are the better competitors tend, on average, to be more resistant to the impacts of consumers.
Fig. 3.
The overall competition–defense correlation (Left) and ranked correlations from individual studies (Right). Negative correlations indicate a tradeoff between competitive ability and defense. Error bars for the overall correlation represent the 95% confidence interval. For individual correlations, the weight given to each study increases with darker shading.
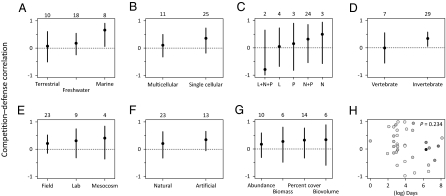
We attempted to determine if measured correlations varied in strength either with the specific type of organism and habitat being considered or with the design of the experiment and how response variables were measured (see Table S1 for a full list of factors considered). However, we were unable to find any associations between ecological or experimental variables and the strength of the competition–defense tradeoff. In fact, most combined correlations remained positive when the dataset was partitioned by category, and there were no significant differences between levels of any categorical effect (Fig. 4 and Tables S2 and S3). There was, however, an association between the abundance hierarchy of plants and the strength of the tradeoff. We partitioned each community into subsets of common and rare species, represented by the four most common and four least common species in each study, respectively. Mean correlations averaged across all studies did not differ between common and rare subsets (P = 0.442; Fig. S4A). In contrast, rare species correlations were consistently lower than those for common species when they were compared within each study (P = 0.045; Fig. S4B), implying that the competition–defense tradeoff is stronger among the rarest species in a community.
Fig. 4.
Competition–defense tradeoffs were not associated with particular ecological traits of studies (A–D) or potential experimental artifacts (E–G). Error bars represent 95% confidence intervals. Sample sizes for categorical variables are given across the top of each panel. Ecological traits: (A) system type; (B) organismal complexity; (C) resource manipulated (L: light; N: nitrogen; P: phosphorous); (D) consumer manipulated. Potential artifacts: (E) study venue; (F) community assembly; (G) response variable; (H) effect of study duration. In H, symbol shading follows Fig. 3.
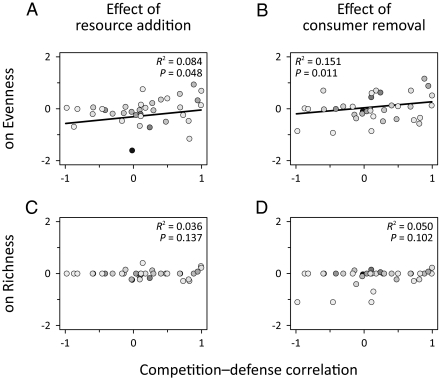
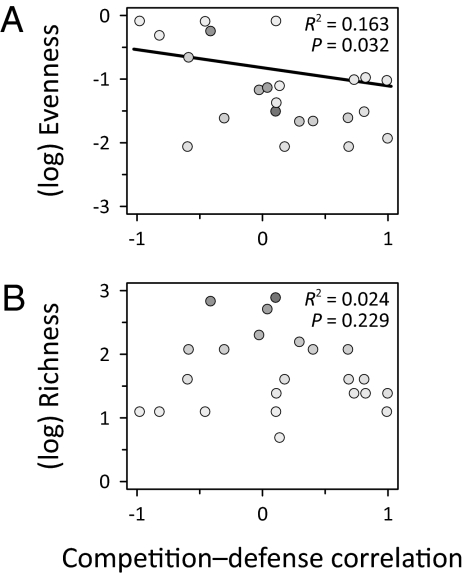
Despite the heterogeneity in correlations over all studies, the relationship between competitive ability and defense did influence species diversity in ways predicted by theory. We measured the change in diversity due to both resource and consumer manipulations, and then determined the relationship between these responses and the competition–defense correlation. Both resource addition and consumer removal decreased species evenness in communities with a competition–defense tradeoff, and increased evenness in communities without a tradeoff (Fig. 5 A and B). Both manipulations also tended to decrease species richness when a competition–defense tradeoff was present, but increase richness when the tradeoff was not present, although these effects were nonsignificant (Fig. 5 C and D). We also found that in the unmanipulated communities, species evenness was greater in systems with a competition–defense tradeoff than in those without (Fig. 6A). There was no significant association between the competition–defense tradeoff and richness (Fig. 6B).
Fig. 5.
Experimental manipulations decrease evenness in communities with competition–defense tradeoffs and increase evenness in communities without tradeoffs. (A and B) Evenness and (C and D) richness responses to resource addition and consumer removal as functions of the competition–defense correlation. Diversity responses are the log response ratios of diversity in manipulated relative to unmanipulated communities. R2 and P values are from weighted linear regressions. Best fit lines for models significant at the α = 0.05 level are shown. Symbol shading follows Fig. 3.
Fig. 6.
Species diversity tends to higher in communities with a competition–defense tradeoff. (A) Evenness and (B) richness in unmanipulated communities. R2 and P values are from weighted linear regressions. Best fit lines for models significant at the α = 0.05 level are shown. Symbol shading follows Fig. 3.
Discussion
We found varied empirical support for a tradeoff between plant species’ ability to compete for resources and their defense against consumers. A slight majority of studies in our analyses showed a pattern opposed to the expected tradeoff: strong competitors tended to be more resistant to consumers. However, competition–defense correlations were highly variable overall and were evident in a minority of systems. Regardless of whether competition–defense correlations were negative or positive, the relationship between competitive ability and defense affected diversity in ways that are consistent with ecological theory. Diversity tended to be higher in studies with negative competition–defense correlations than in those with positive correlations, suggesting that the competition–defense tradeoff does function as a diversity maintenance mechanism where it occurs. For communities with a tradeoff, adding resources or removing consumers reduced diversity because the factors preventing individual species from becoming superabundant had been removed. For communities with positive competition–defense correlations, resource addition and consumer removal tended to increase diversity through differential benefits to species that were both poor resource competitors and poor at withstanding predators. Thus, though competition–defense tradeoffs were not as common as has been assumed previously, when that tradeoff is present, the main predictions of how resources and consumers interactively influence species diversity were supported by this dataset.
Potential Limitations.
Like all meta-analyses, several limitations of our dataset should be kept in mind when interpreting the results. First, our analyses assume that investigators correctly identified the resources and consumers that most limited populations in a given system. Though we cannot verify conclusively that this assumption is valid, additional analyses offer strong support for it. Both resource addition and consumer removal increased community biomass relative to unmanipulated conditions (mean log response ratio for resource addition = 0.47, 95% CI: 0.15–0.79; mean log response ratio for consumer removal = 0.31, 95% CI: 0.14–0.49), which suggests that the resources and consumers manipulated were in fact limiting species’ population growth.
Our conclusions may also be constrained by the species composition that investigators selected for their focal communities. For example, a tradeoff within a particular subset of species may have been masked by the inclusion of additional species differentiated along other niche axes (i.e., those not associated with shared resources or consumers, such as might occur for species that coexist through a competition–colonization tradeoff). Alternatively, species that are poor at both resource acquisition and defense, and thus that are being slowly excluded, may likewise obscure the detection of a tradeoff within the subset of species that stably coexist. Our observation that competition–defense correlations are more negative for the rarest species in a community provides evidence that the strength of a tradeoff can differ between subsets of ecologically similar species. This finding may reflect the fact that species less subject to a competition–defense tradeoff, and therefore capable of being both resistant to consumers and good resource competitors, become more common than those species constrained by the tradeoff.
Alternatives to the Competition–Defense Tradeoff.
Many mechanisms have been proposed to explain the maintenance of species diversity, so it should not be a surprise that the competition–defense tradeoff is absent from some plant communities. Indeed, tradeoffs in requirements for multiple resources; tradeoffs between competitive ability and colonization ability; and temporal or spatial variation in the environment are just a few additional mechanisms that can stabilize plant species assemblages (reviewed in refs. 1 and 11). In many cases, these coexistence mechanisms may operate in concert with the competition–defense tradeoff, or, for communities lacking a competition–defense tradeoff, they may serve as the primary determinants of plant diversity. Because the competition–defense tradeoff was originally conceived to explain how consumers could maintain the diversity of their prey, we focus our attention on consumer-based mechanisms and ask two important questions. First, why is the competition–defense tradeoff not more prevalent? And second, what mechanisms other than the competition–defense tradeoff could explain the beneficial effects of consumers on species diversity?
We propose a simple explanation for why a competition–defense tradeoff is not more common. The mechanistic basis of this tradeoff stems from a larger body of work on allocation-based tradeoffs at the level of the individual. Given that all individuals have finite resources at their disposal, they must strike a balance between investment in resource acquisition and investment in defense. However, for individual-level allocation tradeoffs to extend to whole communities and thereby maintain diversity, individuals of all species must have a similar resource stock (i.e., allocation constraint) to allocate toward competitive ability or toward defense. Our findings may reflect the fact that all species in a community do not share the same constraints. Alternatively, some traits, such as low nitrogen content in a nitrogen-limited system, may simultaneously confer both increased competitive ability and consumer resistance (21).
How can we reconcile our results with empirical work showing that consumers increase species diversity at lower trophic levels (2, 3, 20)? An emerging paradigm emphasizes that the overlap between species in their resource use and in their consumer assemblages is key to understanding when and how consumers will benefit diversity (22). In communities with a competition–defense tradeoff, shared consumers promote diversity by balancing resource and consumer limitation across species. However, in many cases consumers may regulate diversity through other mechanisms in which competition and predation combine to either promote or undermine species coexistence (22). For example, when consumers are specialists rather than generalists, predation can still maintain diversity in the absence of a tradeoff via a Janzen–Connell mechanism (23, 24). Here, each species supports consumer populations that differentially harm themselves relative to their competitors. Whether the competition–defense tradeoff and other consumer-based coexistence mechanisms extend beyond plants to higher trophic levels is uncertain because complex behavioral traits may underlie competitive ability and resistance. However, Holt et al. (9) have speculated that, for animal prey, increased mobility might simultaneously increase the prey species’ resource uptake rate as well as its predation risk. In such a case, the competition–defense tradeoff could apply equally well to animal species.
Our finding that the competition–defense tradeoff is not widespread in plant communities also has more general implications for future research. Although the tradeoff certainly maintains diversity in some systems, ecologists should not simply presume that predation maintains diversity via this tradeoff without explicitly evaluating species’ competitive ability and defense against consumers. However, regardless of whether a tradeoff is present, the relationship between competition and defense does influence species diversity in expected ways, supporting the theoretical predictions. Continued work quantifying the relationships between competitive ability and defense will add to our knowledge of the mechanisms by which abiotic and biotic factors interact to regulate diversity.
Methods
Data Collection.
Our dataset was compiled from studies identified by searching the ISI Web of Science using the search terms [resourc* or nutrient* or fertili*] and [herbivor* or graz* or consum*]. These were the same strings used in recent meta-analyses of community regulation by consumer and nutrient limitation (20, 25). We also included the publications used in these analyses in our pool of potential studies. Studies were only included in our dataset if they met the following criteria: (i) The experimental community was composed of at least three plant species, the minimum required to obtain meaningful correlations between species’ responses to resource addition and consumer removal. (ii) Resource availability (light, nitrogen, phosphorous, or combinations thereof) was directly manipulated and (iii) consumer abundance was directly manipulated. Studies that compared communities in different habitats that varied naturally in resource availability or consumer pressure were not included because of potential confounding effects. And (iv) authors reported species-specific responses (rather than aggregate community responses) to resource and consumer treatments and corresponding measures in control plots. In some instances, authors reported observations from multiple sites, time periods, or under different experimental conditions. These observations were considered independent studies for our purposes. Ultimately, we incorporated 36 studies from 26 separate publications into our dataset. Citations for these publications are provided in SI Text.
When studies reported multiple observations over time (seven studies), we recorded responses at all time points (Fig. S5) but performed our analyses on the first time point only. For these seven studies, we fit a mixed model to determine if competition–defense correlations changed over time (days since start of treatments, log transformed), with study included as a random effect. There was no effect of time (likelihood-ratio test: χ2 = 0.03, df = 1, P = 0.87). To confirm that the choice of time point did not influence our results, we also conducted our analyses using the final time point and reached the same conclusions.
Data were extracted either from tables or from digitized figures using DigitizeIt 1.5.8. Our primary data were species abundances in both experimental and control treatments (solid and dashed symbols in Fig. 1A, respectively). We then inferred competitive ability and sensitivity to predation as the response of a species to resource addition and consumer removal, respectively (i.e., analogous to the arrows in Fig. 1A; see following section and SI Text). Species richness and evenness (the Evar index) (26) were also calculated. In addition to these quantitative data, categorical information about each study was collected to determine if observed competition–defense tradeoffs varied depending on ecological or experimental factors. A complete list of all variables and their definitions is given in Table S1.
Meta-Analysis.
We quantified species’ responses to resource addition and their responses to consumer removal as the proportional change in abundance between experimental and control treatments using log response ratios (27). To detect a tradeoff using this approach, we estimated the response to resource addition when consumers were unmanipulated and the response to consumer removal when resources were unmanipulated (i.e., the responses are orthogonal; Fig. 1A). A common problem with the log response ratio is that zero values of the response variable cause the log ratio to be undefined, and in our dataset, species were occasionally absent in some treatments. We considered zero values as potentially meaningful responses that should not be eliminated, and we corrected them by adding the lowest value of the response variable observed within a study to all observations for that study. This correction yields a conservative estimate of the log response ratio for species that decline below detection limits or have gone extinct.
To quantify the magnitude of a tradeoff in a given study, we calculated the correlation (i.e., the competition–defense correlation) between species’ responses to resource addition (the inverse of competitive ability) and to consumer removal (the inverse of resistance to consumers) as our effect size metric, with negative correlations indicating a tradeoff (Fig. 1B). We tested whether the competition–defense correlation differed from zero with mixed-effects models (27). Correlation coefficients were z-transformed to improve normality (28) and combined across studies with study included as a random effect (29). We weighted studies by the inverse of the sampling variance of their effect sizes, as recommended by Hedges et al. (27), to account for the greater certainty in studies with less variation. In addition to the main model that estimated the mean correlation across all studies, we fit separate models that included fixed categorical effects (Table S1) to account for variability attributable to the ecological or experimental characteristics of each study. Models were fit using the metafor package (29) in R 2.10.1 (www.R-project.org).
To determine how the strength of the competition–defense tradeoff varied with species’ abundance in the community, we examined the subset of studies that were most speciose (eight or more species, n = 17) and ranked species by their abundance under control conditions. We designated the four most abundant species as common and the four least abundant as rare, and then calculated competition–defense correlations within each study for both abundance classes. We fit a model as above with abundance class as a fixed effect to evaluate if the competition–defense correlation differed between common and rare species across all studies. We also determined if there were consistent differences within each study by fitting a regression between the correlations for common species and those for rare species.
We also investigated how the competition–defense correlation influences community structure in two ways. First, the competition–defense correlation may affect a community's response to experimental manipulations. In communities with a strong competition–defense tradeoff, the balance between resource limitation and consumer pressure maintains diversity. Removing either resource limitation or consumer pressure should therefore diminish diversity relative to control treatments. We again used log response ratios to quantify the proportional change in diversity (richness and evenness) between experimental and control treatments. We then tested for relationships between the competition–defense correlation and these diversity responses in separate linear regressions with each observation weighted by the number of species in the community. Second, we evaluated whether those communities with a strong competition–defense tradeoff are more diverse under control conditions. To test this, we performed linear regressions (weighted as above) of studies’ diversity values in control treatments against their competition–defense correlations. Diversity values were log-transformed to meet the assumption of normality. Communities artificially assembled by researchers were not included in these analyses.
Supplementary Material
Acknowledgments
We thank J. Byrnes, B. Gilbert, K. Lafferty, J. Williams, and two anonymous reviewers for providing valuable comments on the manuscript. This work was supported by the National Science Foundation Graduate Research Fellowship (to J.S.G. and D.V.V.) and Grants DEB 0842009 (to B.J.C.) and DEB 0743365 (to J.M.L.), the Department of Energy Global Change Education Program Fellowship (to S.A.S.), the Department of Defense National Defense Science and Engineering Graduate Fellowship (to J.S.G.), and the University of California, Santa Barbara.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007745107/-/DCSupplemental.
References
- 1.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 2.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 3.Lubchenco J. Plant species diversity in a marine intertidal community: Importance of herbivore food preference and algal competitive abilities. Am Nat. 1978;112:23–39. [Google Scholar]
- 4.Worm B, Lotze HK, Hillebrand H, Sommer U. Consumer versus resource control of species diversity and ecosystem functioning. Nature. 2002;417:848–851. doi: 10.1038/nature00830. [DOI] [PubMed] [Google Scholar]
- 5.McCauley E, Briand F. Zooplankton grazing and phytoplankton species richness: Field tests of the predation hypothesis. Limnol Oceanogr. 1979;24:243–252. [Google Scholar]
- 6.Morin PJ. Predation, competition, and the composition of larval anuran guilds. Ecol Monogr. 1983;53:119–138. [Google Scholar]
- 7.Menge BA, Berlow EL, Blanchette CA, Navarrete SA, Yamada SB. The keystone species concept: Variation in interaction strength in rocky intertidal habitat. Ecol Monogr. 1994;64:249–286. [Google Scholar]
- 8.Armstrong RA. Prey species replacement along a gradient of nutrient enrichment: A graphical approach. Ecology. 1979;60:76–84. [Google Scholar]
- 9.Holt RD, Grover J, Tilman D. Simple rules for interspecific dominance in systems with exploitative and apparent competition. Am Nat. 1994;144:741–771. [Google Scholar]
- 10.Leibold MA. A graphical model of keystone predators in food webs: Trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat. 1996;147:784–812. [Google Scholar]
- 11.Kneitel JM, Chase JM. Trade-offs in community ecology: Linking spatial scales and species coexistence. Ecol Lett. 2004;7:69–80. [Google Scholar]
- 12.Leibold MA. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am Nat. 1989;134:922–949. [Google Scholar]
- 13.McPeek MA. The consequences of changing the top predator in a food web: A comparative experimental approach. Ecol Monogr. 1998;68:1–23. [Google Scholar]
- 14.Steiner CF. Keystone predator effects and grazer control of planktonic primary production. Oikos. 2003;101:569–577. [Google Scholar]
- 15.Chase J, Leibold M, Simms E. Plant tolerance and resistance in food webs: Community-level predictions and evolutionary implications. Evol Ecol. 2000;14:289–314. [Google Scholar]
- 16.Tilman D. Resource Competition and Community Structure. Princeton, NJ: Princeton Univ Press; 1982. [PubMed] [Google Scholar]
- 17.Levine JM, Brewer JS, Bertness MD. Nutrients, competition and plant zonation in a New England salt marsh. J Ecol. 1998;86:285–292. [Google Scholar]
- 18.Stanley Harpole W, Tilman D. Non-neutral patterns of species abundance in grassland communities. Ecol Lett. 2006;9:15–23. doi: 10.1111/j.1461-0248.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 19.Darcy-Hall TL, Hall SR. Linking limitation to species composition: Importance of inter- and intra-specific variation in grazing resistance. Oecologia. 2008;155:797–808. doi: 10.1007/s00442-007-0948-z. [DOI] [PubMed] [Google Scholar]
- 20.Hillebrand H, et al. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc Natl Acad Sci USA. 2007;104:10904–10909. doi: 10.1073/pnas.0701918104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craine JM, et al. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol. 2002;16:563–574. [Google Scholar]
- 22.Chesson P, Kuang JJ. The interaction between predation and competition. Nature. 2008;456:235–238. doi: 10.1038/nature07248. [DOI] [PubMed] [Google Scholar]
- 23.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 24.Connell JH. In: Dynamics of Populations. den Boer PJ, Gradwell GR, editors. Wageningen, The Netherlands: Pudoc; 1971. pp. 298–312. [Google Scholar]
- 25.Gruner DS, et al. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol Lett. 2008;11:740–755. doi: 10.1111/j.1461-0248.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith B, Wilson JB. A consumer's guide to evenness indices. Oikos. 1996;76:70–82. [Google Scholar]
- 27.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- 28.Caruso JC, Cliff N. Empirical size, coverage, and power of confidence intervals for Spearman's rho. Educ Psychol Meas. 1997;57:637–654. [Google Scholar]
- 29.Viechtbauer W. The metafor package: A meta-analysis package for R. 2009. Available at http://cran.r-project.org/web/packages/metafor/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.