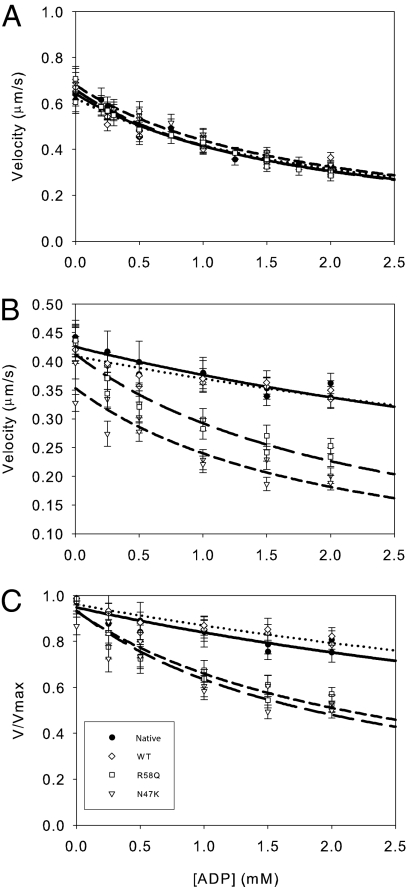
Fig. 3.
Exogenous ADP decreases actin filament velocity. The raw unloaded (A), unnormalized loaded (B), and normalized data (C) are shown with fits to a competitive inhibitor model (Materials and Methods). The inhibition constant is similar for all myosins under unloaded conditions (A) (Table 2). (B and C) Load decreases the sensitivity of actin filament velocity to exogenously added ADP. The unnormalized (B) and normalized (C) data were fit as in A. Loading the myosin causes a significant reduction in the ability of ADP to depress actomyosin sliding for native and WT myosins. Loading both native and WT exchanged myosins causes a lowering of the affinity for exogenously added ADP (Table 2). However, load has minimal effect for the N47K and R58Q mutant myosins with KI values similar to the unloaded case, suggesting a loss of strain sensitivity (Table 2). Native (●, solid line), WT (◇, dotted line), N47K (▽, short-dashed line) and R58Q (□, long-dashed line) are shown.