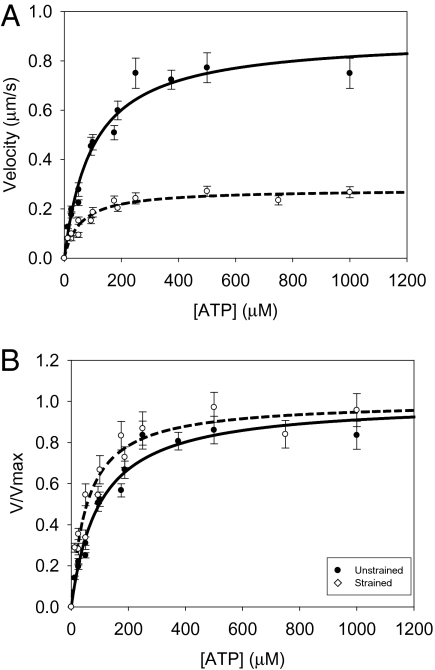
Fig. 4.
Exogenously added ATP increases actin filament velocity. The (A) unnormalized and (B) normalized data are shown with fits to a Michaelis–Menten model. Decreasing the concentration of ATP below 100 μM causes a significant depression in actin filament velocity for both unloaded and loaded myosins. Load increases the sensitivity of actin filament velocity to exogenously added ATP. The KM for unloaded myosin (●, solid line) is significantly higher than that for loaded myosin (○, dashed line; Table 2; P < 0.05). Load was introduced into the motility assay using the low-affinity actin-binding protein, α-actinin. A similar shift was observed for WT myosin (Table 2).