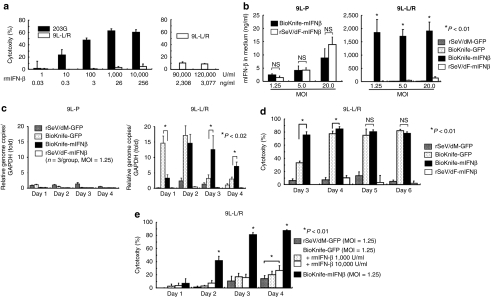
Figure 4.
Drastic synergy induced by BioKnife and mIFN-β gene transfer for killing 9L-L/R cells (*P < 0.01). (a) Direct cytotoxic effect of recombinant murine IFN-β (rmIFN-β) on mouse glioma 203G and 9L-L/R cells. Four days after treatment with various amounts of rmIFN-β, a cytotoxicity assay was performed. Note that 203G cells were susceptible to the rmIFN-β protein and dose-dependent cytotoxicity was observed, whereas 9L-L/R cells were highly resistant to rmIFN-β protein even at a concentration of over 90,000 U/ml (equivalent to 2,308 ng/ml). Each group contains n = 4. (b) Cell fusion via BioKnife enhances its mIFN-β transgene expression on 9L-L/R (susceptible to cell fusion, right graph), but not on 9L-P (resistant to cell fusion, left graph). Four days after exposure to each virus at each MOI, the mIFN-β protein level was measured by specific enzyme-linked immunosorbent assay. No significant difference in expression levels was found between rSeV/dF-mIFNβ (nonfusogenic) and BioKnife-mIFNβ (fusogenic) using 9L-P cells (left graph). Note that over 2-logs higher mIFN-β protein levels were found with BioKnife-mIFNβ than with rSeV/dF-mIFNβ at any MOI in 9L-L/R cells (right graph). Significant, but very low levels of mIFN-β protein were detected when using rSeV/dM-GFP and BioKnife-GFP. Each group contains n = 4. (c) Cell fusion via BioKnife potentiates viral genome copies on 9L-L/R (susceptible to cell fusion, right graph), but not on 9L-P (resistant to cell fusion, left graph). After exposure to each virus at an MOI of 1.25 on each time point, viral genome copies were quantified by real-time reverse transcriptase-PCR targeting of the N gene, their common sequence. Each data point was standardized by simultaneous amplification of GAPDH (the genome copies/GAPDH on day 1 in 9L-P cells treated with rSeV/dM-GFP = 1). Strong and early amplification of genome copies of BioKnife-GFP was found in 9L-L/R cells, and declined 3 days after infection. In contrast, delayed and sustained increase of genome copies was found when using BioKnife-mIFNβ. Each group contains n = 3. (d) Arming with the mIFN-β gene accelerates the cytotoxicity of BioKnife to mIFN-β-insensitive 9L-L/R cells. At various time points after exposure to each virus at an MOI of 1.25, a cytotoxicity assay was performed. Note that BioKnife-GFP showed peak cytotoxicity from day 4, whereas BioKnife-mIFNβ had already reached its peak cytotoxicity on day 3. Each group contains n = 6. (e) Acceleration of cell fusion and cytotoxicity due to BioKnife is efficiently induced by mIFN-β gene transfer, but not by rmIFN-β protein. At various time points after exposure to each virus at an MOI of 1.25, a cytotoxicity assay was performed. Note that a modest but significant acceleration of cytotoxicity via BioKnife-GFP was found only in the presence of a high concentration of rmIFN-β (10,000 U/ml) on day 4. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MOI, multiplicity of infection; NS, nonsignificant; rmIFN-β, recombinant murine interferon-β.