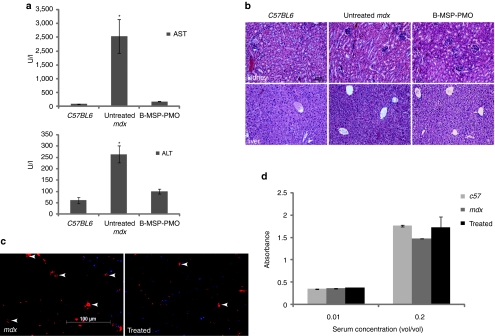
Figure 4.
Investigation of systemic immunotoxicity in mdx mice treated with the multiple B-MSP-PMO conjugate administration. (a) Measurement of serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes in treated mdx mice compared with untreated mdx mice. Data show a improved pathological parameters in B-MSP-PMO-treated mdx mice compared with untreated controls with significantly lower serum levels of both enzymes (t-test, *P < 0.05; six mice each group). (b) Hematoxylin and eosin staining of kidney (upper panel) and liver (lower panel) tissues sections from treated mdx mice with B-MSP-PMO, untreated mdx mice, and C57BL6 normal controls. Bar = 200 µm. Tissue sections from renal cortex show normal glomerular and tubule tissue architecture in B-MSP-PMO treated and control mice. No difference was observed for B-MSP-PMO treated mdx mice and untreated mdx controls in liver sections. (c) Detection of CD3+ T lymphocytes in the diaphragms of treated and untreated mdx mice. Bar = 100 µm. Arrows indicate the T lymphocytes detected by CD3 mouse monoclonal antibody. (d) ELISA results to detect specific antibody against the B-MSP-PMO compound in the serum from the treated mdx mice at 6 mg/kg biweekly dosing regimens. No difference was observed between treated and untreated mdx mice (six mice each group). ELISA, enzyme-linked immunosorbent assay; MSP, muscle-specific heptapeptide; PMO, phosphorodiamidate morpholino.