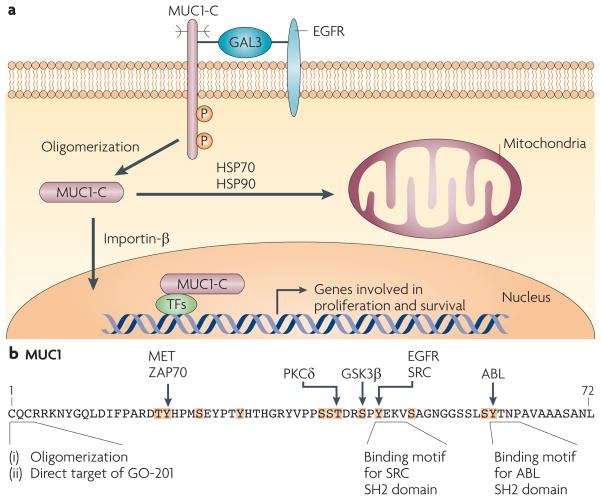
Figure 5. Activation of MUC1-C in stress and transformation.
a| The mucin 1 (MUC1) C-terminal transmembrane subunit (MUC1-C) forms complexes with the epidermal growth factor receptor (EGFR) that are mediated at least in part by galectin 3 (GAL3) lattices. MUC1-C also forms complexes with ERBB2–4 and with other receptor tyrosine kinases (not shown), although it is not known whether these interactions require galectin 3. MUC1-C–RTK complexes are transiently formed in non-malignant epithelial cells in response to inflammatory cytokines and are constitutively formed in carcinoma cells. MUC1-C oligomers are targeted to the nucleus by an importin-β-dependent mechanism, where MUC1-C associates with several transcription factors (TFs)77. MUC1-C thereby promotes the transcription of genes that encode proteins involved in proliferative and pro-survival signals by affecting the recruitment of co-activators and co-repressors93,125 and/or regulating the latency of transcription factor occupancy73. MUC1-C is also targeted to the mitochondrial outer membrane by heat shock protein 70 (HSP70) and HSP90, where it blocks stress-induced loss of the mitochondrial transmembrane potential28,29,89 and the induction of cell death in response to DNA damage, reactive oxygen species, hypoxia and glucose deprivation28,150–153. b | MUC1-C intracellular signalling is mediated in part by phosphorylation of the cytoplasmic domain, which contains five documented and seven potential phosphorylation sites (highlighted in orange with known kinases indicated). The CQC sequence is necessary for oligomerization of MUC1-C and targeting of MUC1-C to the nucleus and mitochondria, as well as binding of MUC1-C to diverse effectors, such as nuclear factor-κB73. Direct targeting of the MUC1-C CQC sequence with GO-201 induces complete regression of established human breast and prostate tumour xenografts in mice81,82. GSK3β, glycogen synthase kinase 3β; P, phosphorylation; PKCδ, protein kinase Cδ; SH2, Src homology 2.