Abstract
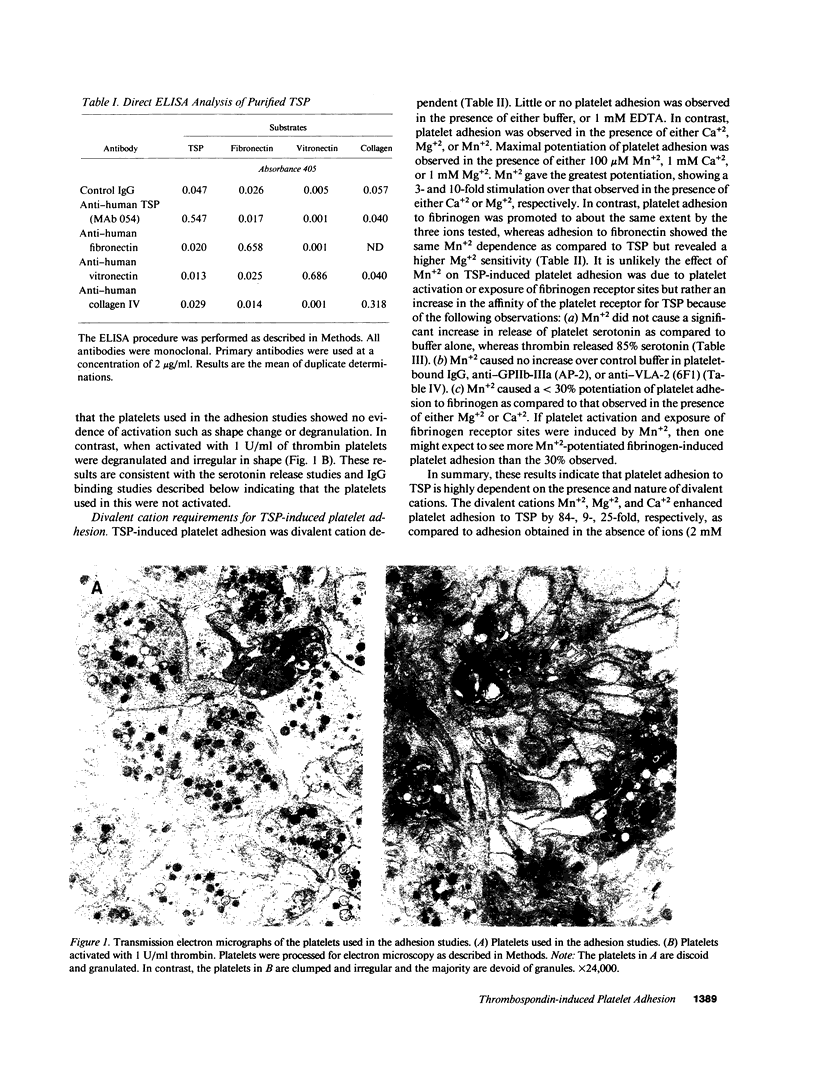
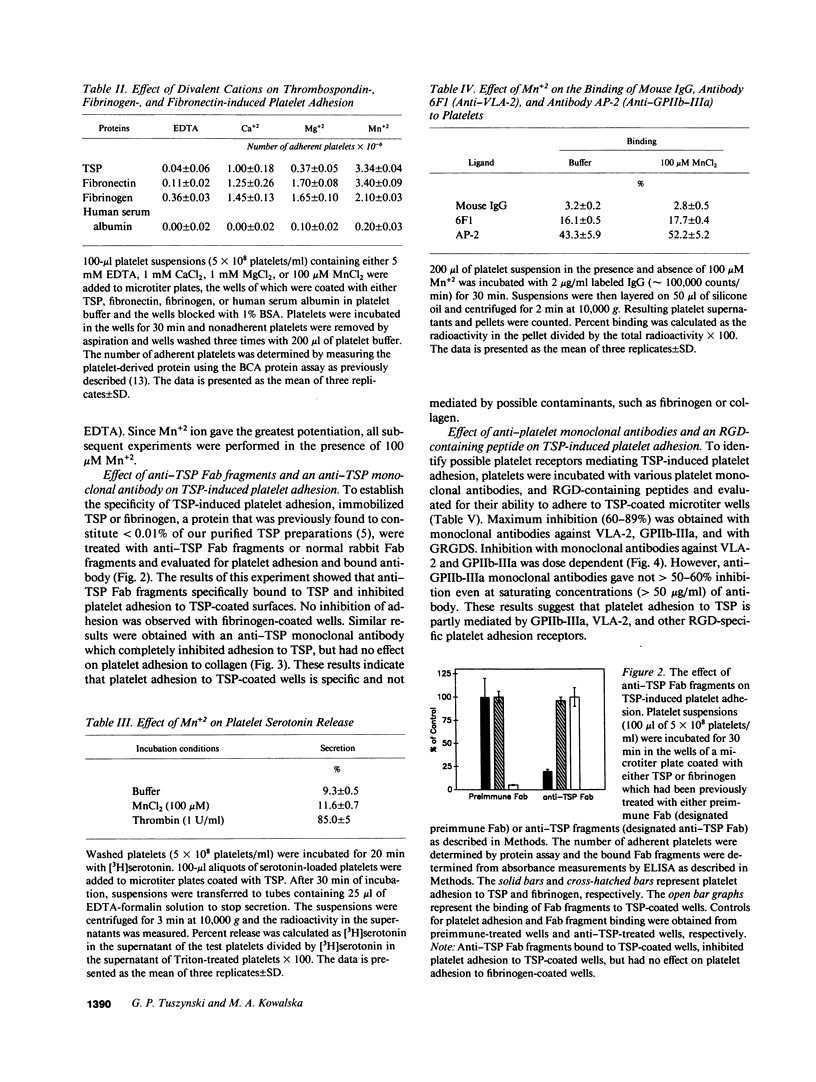
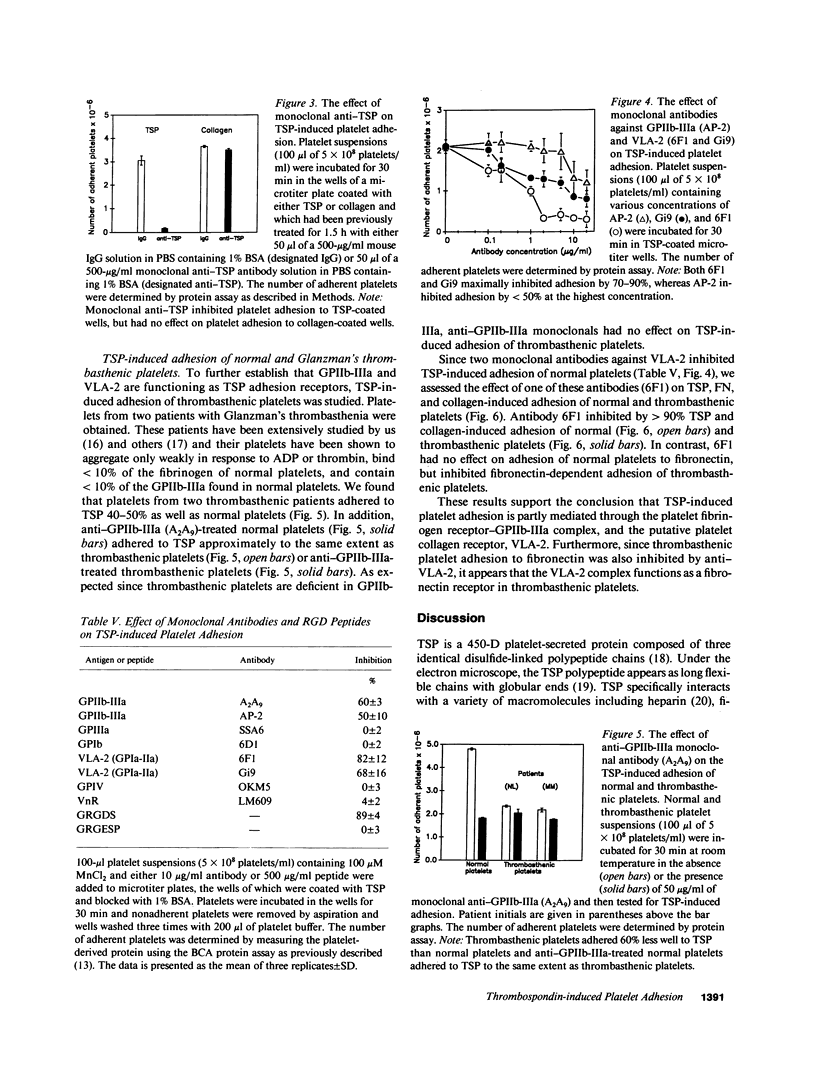
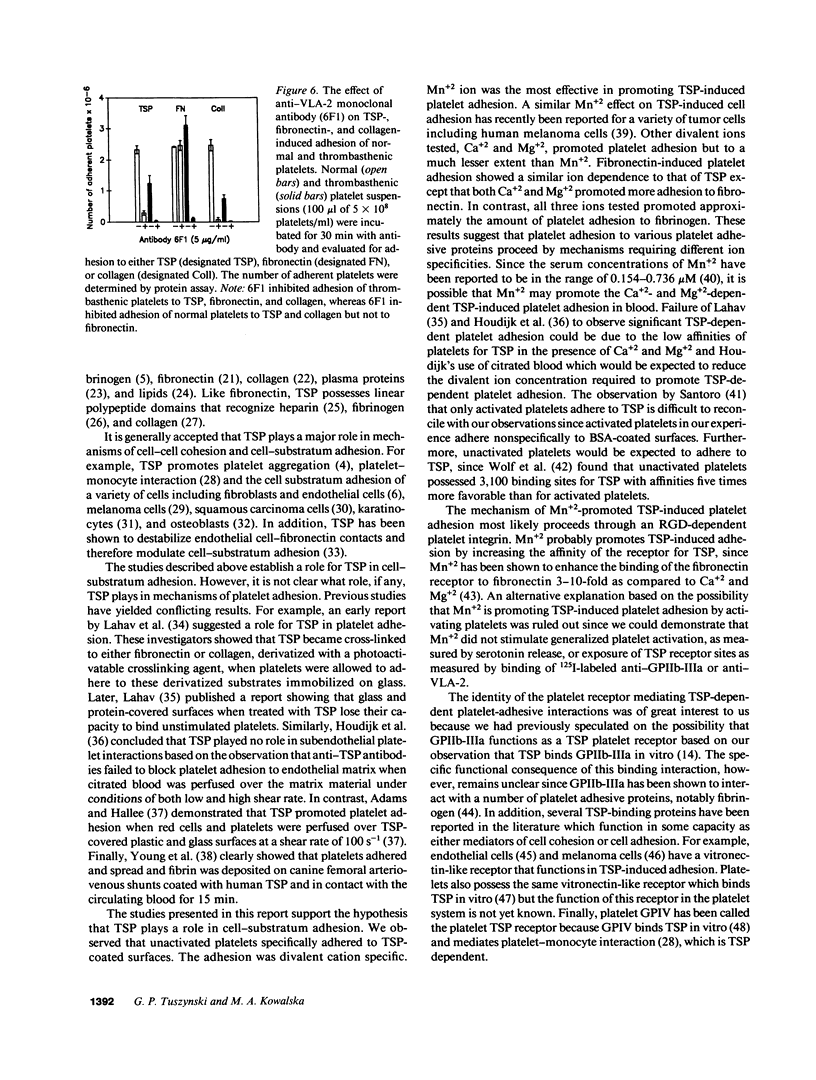
Washed human unactivated platelets attached and spread on thrombospondin (TSP)-coated microtiter plates. Platelet adhesion was promoted by divalent cations Mn2+, Mg2+, and Ca2+ as compared to buffer having all divalent cations complexed with EDTA. TSP-dependent adhesion was inhibited by anti-TSP fab fragments, an anti-TSP monoclonal antibody, an RGD-containing peptide, complex-specific anti-glycoprotein (GP)IIb-IIIa monoclonal antibodies (A2A9 or AP-2) and anti-VLA-2 monoclonal antibodies (6F1 and Gi9), but not by rabbit preimmune fab fragments, mouse IgG, an anti-GPIIIa monoclonal antibody, or monoclonal antibodies against either the human vitronectin receptor, glycocalicin, or GPIV. At saturating concentrations, anti-GPIIb-IIIa inhibited adhesion by 40-60%. Glanzman's thrombasthenic platelets, which lack GPIIb-IIIa, adhered to TSP to the same extent as anti-GPIIb-IIIa-treated normal platelets or 40-60% as well as untreated normal platelets. Antibody 6F1 (5-10 micrograms/ml) inhibited platelet adhesion of both normal and thrombasthenic platelets by 84-100%. Both VLA-2 antibodies also inhibited collagen-induced platelet adhesion, but had no effect on fibronectin-induced adhesion of normal platelets. These data indicate that platelets specifically adhere to TSP and that this adhesion is mediated through GPIIb-IIIa and/or VLA-2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruthio F., Guillard O., Arnaud J., Pierre F., Zawislak R. Determination of manganese in biological materials by electrothermal atomic absorption spectrometry: a review. Clin Chem. 1988 Feb;34(2):227–234. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Clezardin P., McGregor J. L., Lyon M., Clemetson K. J., Huppert J. Characterization of two murine monoclonal antibodies (P10, P12) directed against different determinants on human blood platelet thrombospondin. Eur J Biochem. 1986 Jan 2;154(1):95–102. doi: 10.1111/j.1432-1033.1986.tb09363.x. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Day H. J., Rao A. K. Evaluation of platelet function. Semin Hematol. 1986 Apr;23(2):89–101. [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Dixit V. M., Hennessy S. W., Grant G. A., Rotwein P., Frazier W. A. Characterization of a cDNA encoding the heparin and collagen binding domains of human thrombospondin. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5449–5453. doi: 10.1073/pnas.83.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin N. J., Vance P. M., Dixit V. M., Fink B., Frazier W. A. Interaction of human thrombospondin with types I-V collagen: direct binding and electron microscopy. J Cell Biol. 1987 May;104(5):1413–1422. doi: 10.1083/jcb.104.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautanen A., Gailit J., Mann D. M., Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989 Jan 25;264(3):1437–1442. [PubMed] [Google Scholar]
- Houdijk W. P., de Groot P. G., Nievelstein P. F., Sakariassen K. S., Sixma J. J. Subendothelial proteins and platelet adhesion. von Willebrand factor and fibronectin, not thrombospondin, are involved in platelet adhesion to extracellular matrix of human vascular endothelial cells. Arteriosclerosis. 1986 Jan-Feb;6(1):24–33. doi: 10.1161/01.atv.6.1.24. [DOI] [PubMed] [Google Scholar]
- Karczewski J., Knudsen K. A., Smith L., Murphy A., Rothman V. L., Tuszynski G. P. The interaction of thrombospondin with platelet glycoprotein GPIIb-IIIa. J Biol Chem. 1989 Dec 15;264(35):21322–21326. [PubMed] [Google Scholar]
- Kehrel B., Balleisen L., Kokott R., Mesters R., Stenzinger W., Clemetson K. J., van de Loo J. Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen-induced aggregation and spontaneous loss of disorder. Blood. 1988 Apr;71(4):1074–1078. [PubMed] [Google Scholar]
- Lahav J., Lawler J., Gimbrone M. A. Thrombospondin interactions with fibronectin and fibrinogen. Mutual inhibition in binding. Eur J Biochem. 1984 Nov 15;145(1):151–156. doi: 10.1111/j.1432-1033.1984.tb08534.x. [DOI] [PubMed] [Google Scholar]
- Lahav J., Schwartz M. A., Hynes R. O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982 Nov;31(1):253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lahav J. Thrombospondin inhibits adhesion of platelets to glass and protein-covered substrata. Blood. 1988 Apr;71(4):1096–1099. [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lawler J., Derick L. H., Connolly J. E., Chen J. H., Chao F. C. The structure of human platelet thrombospondin. J Biol Chem. 1985 Mar 25;260(6):3762–3772. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989 Nov 1;74(6):2022–2027. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L., Harpel P. C. Complex formation of platelet thrombospondin with histidine-rich glycoprotein. J Clin Invest. 1984 Jan;73(1):5–12. doi: 10.1172/JCI111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. M., Wehring B. Isoelectric characteristics and surface radioiodination of normal and thrombasthenic platelet membrane glycoproteins. Thromb Res. 1981 Apr 1;22(1-2):53–65. doi: 10.1016/0049-3848(81)90308-x. [DOI] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Prochownik E. V., O'Rourke K., Dixit V. M. Expression and analysis of COOH-terminal deletions of the human thrombospondin molecule. J Cell Biol. 1989 Aug;109(2):843–852. doi: 10.1083/jcb.109.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser B. L., Varani J., O'Rourke K., Dixit V. M. Thrombospondin binding by human squamous carcinoma and melanoma cells: relationship to biological activity. Exp Cell Res. 1988 Feb;174(2):319–329. doi: 10.1016/0014-4827(88)90303-5. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Haverstick D. M., Dixit V. M., Frazier W. A., Santoro S. A., Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem. 1985 Aug 5;260(16):9405–9411. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Fisher L. W., McClain T. D. Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J Cell Biol. 1989 Feb;108(2):719–727. doi: 10.1083/jcb.108.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A. Thrombospondin and the adhesive behavior of platelets. Semin Thromb Hemost. 1987 Jul;13(3):290–297. doi: 10.1055/s-2007-1003504. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Asch A. S., Nachman R. L. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 1989 Aug;84(2):546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalska H. I., Niewiarowski S., Tuszynski G. P., Rucinski B., Schmaier A. H., Morinelli T. A., Cierniewski C. S. Radioimmunoassay of human platelet thrombospondin: different patterns of thrombospondin and beta-thromboglobulin antigen secretion and clearance from the circulation. J Lab Clin Med. 1985 Dec;106(6):690–700. [PubMed] [Google Scholar]
- Tuszynski G. P., Karczewski J., Smith L., Murphy A., Rothman V. L., Knudsen K. A. The GPIIB-IIIa-like complex may function as a human melanoma cell adhesion receptor for thrombospondin. Exp Cell Res. 1989 Jun;182(2):473–481. doi: 10.1016/0014-4827(89)90251-6. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L., Piperno J. R., Walsh P. N. A rapid method for removal of [125I]iodide following iodination of protein solutions. Anal Biochem. 1980 Jul 15;106(1):118–122. doi: 10.1016/0003-2697(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Mauco G. P., Koshy A., Schick P. K., Walsh P. N. The platelet cytoskeleton contains elements of the prothrombinase complex. J Biol Chem. 1984 Jun 10;259(11):6947–6951. [PubMed] [Google Scholar]
- Tuszynski G. P., Murphy A. Spectrophotometric quantitation of anchorage-dependent cell numbers using the bicinchoninic acid protein assay reagent. Anal Biochem. 1990 Jan;184(1):189–191. doi: 10.1016/0003-2697(90)90032-5. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V. L., Murphy A., Siegler K., Knudsen K. A. Thrombospondin promotes platelet aggregation. Blood. 1988 Jul;72(1):109–115. [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V., Murphy A., Siegler K., Smith L., Smith S., Karczewski J., Knudsen K. A. Thrombospondin promotes cell-substratum adhesion. Science. 1987 Jun 19;236(4808):1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Srivastava S., Switalska H. I., Holt J. C., Cierniewski C. S., Niewiarowski S. The interaction of human platelet thrombospondin with fibrinogen. Thrombospondin purification and specificity of interaction. J Biol Chem. 1985 Oct 5;260(22):12240–12245. [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff R., Plow E. F., Ginsberg M. H. Interaction of thrombospondin with resting and stimulated human platelets. J Biol Chem. 1986 May 25;261(15):6840–6846. [PubMed] [Google Scholar]
- Young B. R., Doyle M. J., Collins W. E., Lambrecht L. K., Jordan C. A., Albrecht R. M., Mosher D. F., Cooper S. L. Effect of thrombospondin and other platelet alpha-granule proteins on artificial surface-induced thrombosis. Trans Am Soc Artif Intern Organs. 1982;28:498–503. [PubMed] [Google Scholar]