Table 1.
Optimization of the MAP reaction with acetals
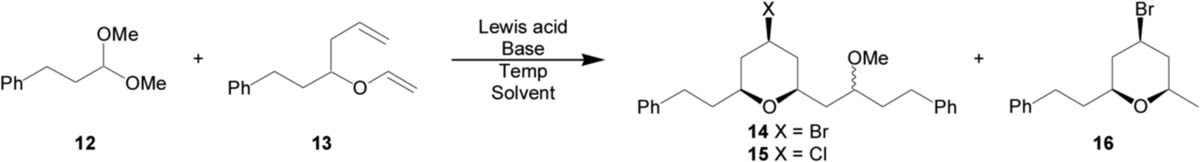 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Lewis Acida | Base (equiv) | Temp | Solvent | Yield | dr (X) eq:axb | dr (OMe)c |
| 1d | TiBr4 | None | −78 °C | CH2Cl2 | 43% | 86:14 | 55:45 |
| 2d |
TiBr4 |
iPr2NEt (1.5) |
−78 °C |
CH2Cl2 |
29% |
82:18 |
55:45 |
| 3 |
TiBr4 |
2,6-DBMP (1.5) |
−78 °C |
CH2Cl2 |
67% |
92:8 |
55:45 |
| 4 | TiBr4 | 2,6-DBMP (1.5) | −78 °C | CH2Cl2/hexanes | 44% | 93:7 | 55:45 |
| 5 | TiBr4/Ti(OiPr)4e | 2,6-DBMP (1.5) | −78 °C | CH2Cl2 | 50% | 91:9 | 53:47 |
| 6 | TiCl4 | 2,6-DBMP (1.5) | −78 °C | CH2Cl2 | 44% | 90:10 | 57:43 |
| 7 | TMSBr | 2,6-DBMP (1.5) | 0 °C | CH2Cl2 | 0% | – | – |
| 8 | AlBr3f | AlMe3 (0.3) | −40 °C | CH2Cl2 | 40% | 80:20 | 56:44 |
Unless otherwise noted, 4 equiv of Lewis acid was used.
Determined by integration of the crude 1H NMR spectra.
Determined by GC analysis.
17% of THP 16 was also isolated.
Formed by pre-mixing an 8:1 solution of TiBr4 and Ti(OiPr)4.
1.5 equiv of AlBr3.
