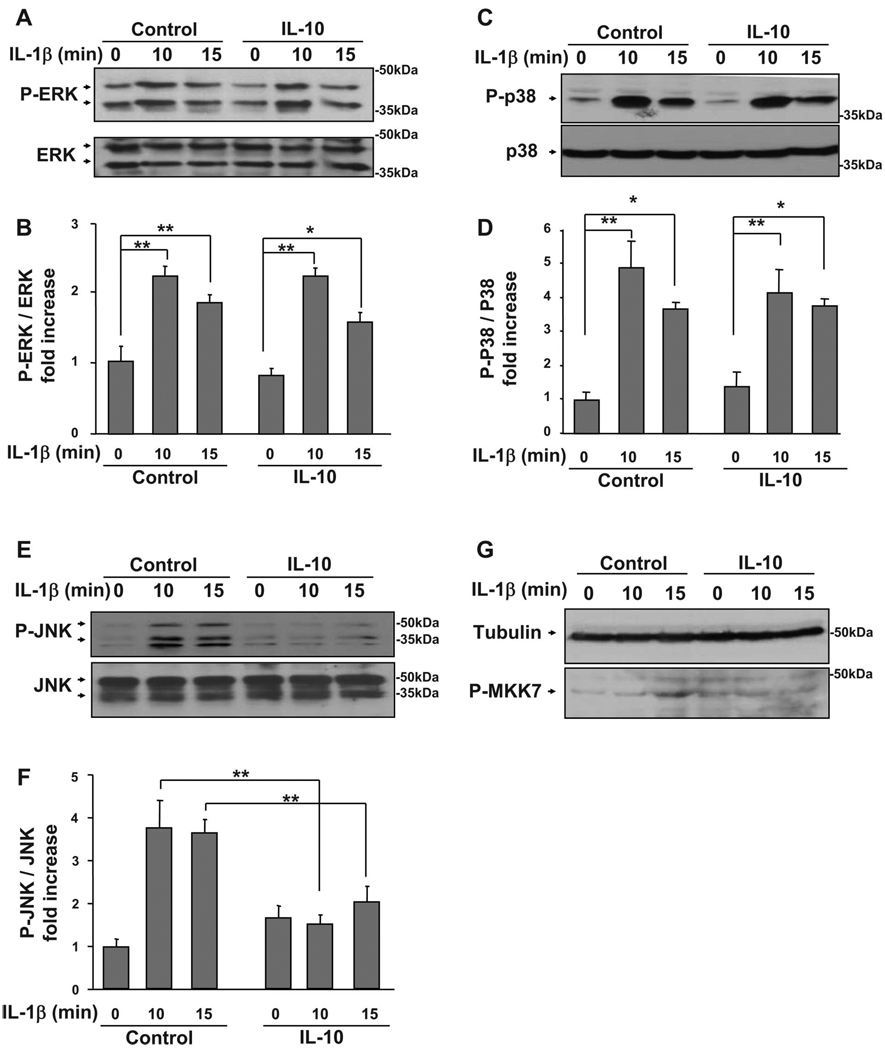
Fig. 4.
IL-10 blocks IL-1β-induced phosphorylation of JNK but not ERK1/2 or p38. C2C12 myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) for another 10 and 15 min. Phosphorylation of ERK1/2, p38, and JNK was determined as in Fig. 3. Membranes were stripped and reblotted with antibodies specific for total ERK1/2, p38, or JNK to ensure that expression of these proteins does not change. Phosphorylation of MKK7 was determined as described in the materials and methods. Densitometric summaries were calculated as ratios of phospho-ERK1/2 to ERK1/2, phospho-p38 to p38, phospho-JNK to JNK, and phospho-MKK7 to α-tubulin. Representative Western blots (A) and a densitometric summary (B) of 6 independent experiments demonstrated that IL-10 does not inhibit IL-1β-induced phosphorylation of ERK1/2. Similarly, IL-10 did not inhibit IL-1β-induced phosphorylation of p38. C: representative Western blots. D: densitometric summary of 3 independent experiments. Conversely, IL-10 completely blocked the IL-1β-induced increase in JNK phosphorylation, as demonstrated by representative Western blots (E) and a quantitative summary (F) of 5 independent experiments. G: similarly, IL-10 suppressed IL-1β-induced MKK7 phosphorylation; representative Western blot of 3 independent experiments. *P < 0.05; **P < 0.01.