Figure 4.
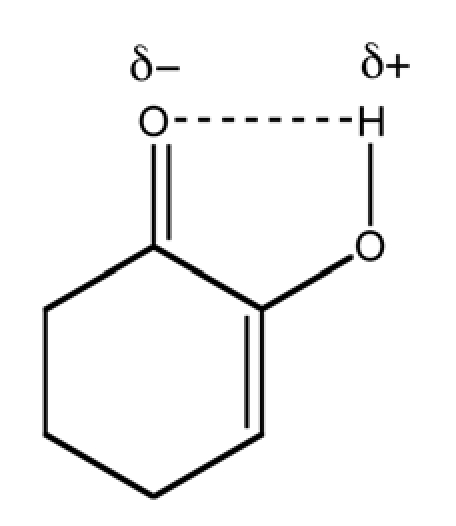
Enol tautomer of cyclohexane-1,2-dione. Although the keto form is generally favored over the enol tautomer, in the case of “4,” hydrogen bonding of enol with the carbonyl results in increased contribution of enol to the equilibrium state.

Enol tautomer of cyclohexane-1,2-dione. Although the keto form is generally favored over the enol tautomer, in the case of “4,” hydrogen bonding of enol with the carbonyl results in increased contribution of enol to the equilibrium state.