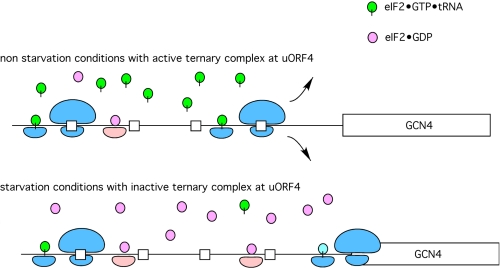
FIGURE 2.
Scanning at GCN4 mRNA. Under normal conditions, the product of the initiation pathway, eIF2·GDP, is recycled to eIF2·GTP by the aid of a guanine nucleotide exchange factor, eIF2B. With high levels of the ternary complex available (eIF2·GTP·tRNAiMet), ribosomes that translate uORF1 will rapidly reacquire a new ternary complex and initiate at uORF4, followed by complete release of the ribosomal subunits from the mRNA and no synthesis of Gcn4. Under conditions of starvation, uncharged tRNA activates Gcn2, leading to phosphorylation of the α-subunit of eIF2. This phosphorylated form of eIF2 complexed with GDP binds tightly to the nucleotide exchange factor and thereby blocks nucleotide exchange, resulting in low levels of ternary complexes. The absence of a large pool of ternary complexes delays the pairing of the ternary complex with an AUG start codon until the AUG for Gcn4 is reached (i.e. the upstream uORFs are bypassed). This results in an increase in Gcn4 expression relative to normal or non-starvation conditions.