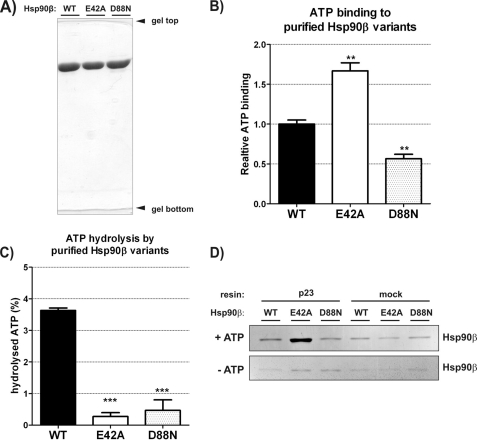
FIGURE 1.
In vitro ATP binding, hydrolysis, and interaction with p23 by human Hsp90β variants: WT, E42A, and D88N. A, 10% SDS-PAGE result demonstrating the purity of Hsp90β variants used in studies in vitro. B, ATP binding efficiency by WT, E42A, and D88N Hsp90β, detected by α-radiolabeled ATP (described under “Experimental Procedures”), shown as a fraction of WT Hsp90β. Each bar is a mean of three experiments, standard errors are shown. **, indicates a statistical significance (p < 0.01) in one-way ANOVA test (Dunnett post-test against the WT Hsp90β result). Bars have been corrected for background binding of ATP to 20-fold excess of BSA, present also in the reactions containing Hsp90β variants. Background was 16% of the WT Hsp90β result. C, ATP hydrolysis efficiency by WT, E42A, and D88N Hsp90β, shown as a fraction of a hydrolyzed γ-radiolabeled ATP, during a 3-h reaction, at 37 °C. Results were corrected for the background ATPase activity, remaining in control samples of all Hsp90β variants after inhibition by radicicol (see “Experimental Procedures”). Each bar is a mean of three experiments, and standard errors are shown. ***, indicates a statistical significance (p < 0.001) in one-way ANOVA test (Dunnett post-test against the WT Hsp90β result). D, silver-stained SDS-PAGE gel showing indicated Hsp90β variants eluted from the complex with p23-conjugated or mock resin, in the presence or absence of 5 mm ATP (see “Experimental Procedures”). Mock controls contained no p23 protein conjugated to the resin. The interaction with p23 co-chaperone is the most efficient in the case of E42A Hsp90β in the presence of ATP.