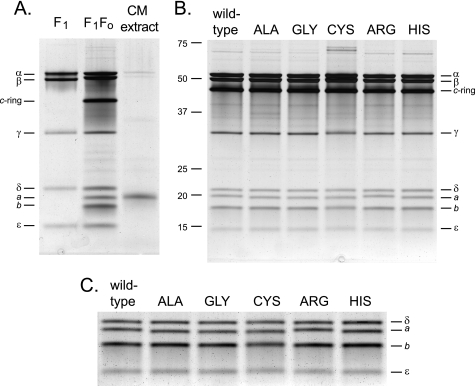
FIGURE 3.
SDS-PAGE analysis of purified ATP synthase preparations from the wild-type and single Lys-180 mutant strains. A, the identity of the a-subunit band was determined on a wild-type ATP synthase sample by an acidified chloroform/methanol extraction, which has been shown to extract both a- and c-subunits (48). The extract is shown in the right lane; for comparison, the purified B. pseudofirmus OF4 F1 is shown on the left, and the purified F1F0 is in the middle lane. B, patterns for each of the mutant enzymes side-by-side with the wild-type preparation. The ATP synthases were purified as detailed under “Experimental Procedures.” The samples (2 μg) were resolved on 11% gels and silver stained. C, to facilitate comparison of the a-subunit bands, the region of the small subunits from the gel in B was cropped out, subjected to auto levels in Adobe Photoshop to intensify the bands, and enlarged. The bromphenol blue front was run off the gels in A and B to optimize resolution of the δ- and a-subunits; as a consequence, trace amounts of c-monomer in samples in B and the substantial amount of c-monomer in the chloroform/methanol extract in A were not visualized. The F1 preparation in A is not His-tagged, and therefore its β-subunit migrated more rapidly than its counterpart in the F1F0 preparation.