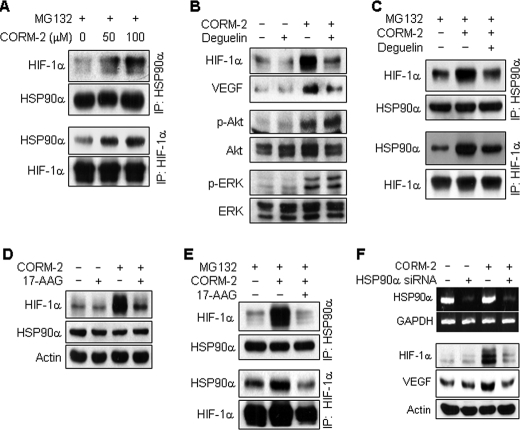
FIGURE 5.
CORM-2 increases HIF-1α protein stability by promoting its interaction with HSP90α. A, astrocytes were treated with or without CORM-2 in the presence of MG132 (10 μm) for 4 h, and cell extracts were subjected to immunoprecipitation (IP) using antibodies against HSP90α and HIF-1α. Immunoprecipitates were separated by electrophoresis, and protein levels of HIF-1α and HSP90 were determined by Western blotting. B, astrocytes were pretreated with 100 nm deguelin for 15 min and incubated with or without CORM-2 (100 μm) for 8 h. The levels of HIF-1α, VEGF, phospho-Akt (p-Akt), and phospho-ERK (p-ERK) were determined by Western blot analyses. C, astrocytes pretreated with 100 nm deguelin for 15 min were incubated with or without CORM-2 (100 μm) in the presence of MG132 (10 μm) for 4 h. Cell extracts were subjected to immunoprecipitation using antibodies against HSP90α and HIF-1α. Immunoprecipitates were separated by electrophoresis, and the levels of HIF-1α or HSP90 were determined by Western blotting. D, cells pretreated with or without 10 μm 17-AAG for 15 min were incubated with or without CORM-2 (100 μm) for 8 h, and the levels of HIF-1α or HSP90 were determined by Western blotting. E, cells pretreated with 10 μm 17-AAG for 15 min and incubated with or without CORM-2 (100 μm) in the presence of MG132 (10 μm) for 4 h. Cell extracts were subjected to immunoprecipitation using an antibody against HSP90α or HIF-1α. Immunoprecipitates were analyzed by Western blotting with antibodies against HIF-1α and HSP90. F, cells were transfected with control or HSP90 siRNA (75 nm), cultured in fresh medium for 40 h, and then treated with or without CORM-2 (100 μm) for 8 h. HSP90α and GAPDH mRNA levels were determined by RT-PCR, and protein levels of HIF-1α and VEGF were detected by Western blot analyses.