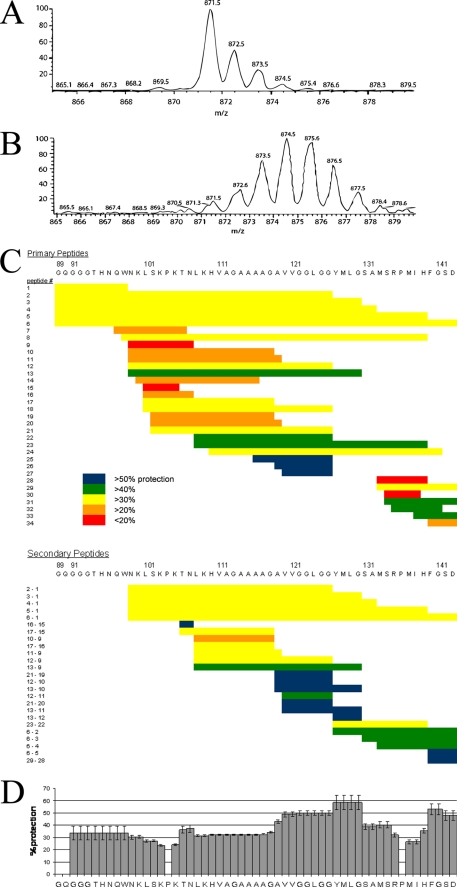
FIGURE 1.
Exchange behavior of PrP(89–143, P101L) fibrils observed by mass spectrometry. A, shows the mass envelope of the 1+ charge state of the proteolytic fragment of PrP(89–143, P101L) corresponding to residues 133–139. B, mass envelope of the same peptide obtained from PrP fibrils that had been incubated in D2O for 1 week. C, exchange behavior of proteolytic fragments of PrP(89–143, P101L) after 6 weeks of incubation in D2O. The extent of exchange is depicted as a percent protection of exchangeable backbone amides. Each primary peptide is numbered. The identity of the two primary peptides used to obtain each secondary peptide is indicated to the left of the secondary peptides. D, consensus exchange behavior calculated according to Equation 1.