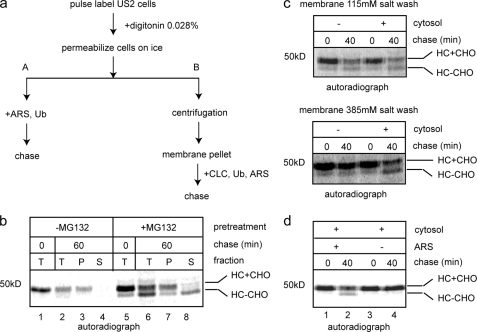
FIGURE 2.
A permeabilized cell assay for US2-mediated retrotranslocation. a, experimental schemes for the permeabilized cell assay. After cells are permeabilized, they were either incubated with an ARS and ubiquitin (Ub) directly to initiate retrotranslocation (scheme A) or subjected to centrifugation to remove cytosol (scheme B). In the latter case, the membrane pellet fraction was washed with salt before the addition of CLC, Ub, and ARS. b, US2 cells were either untreated or treated with the proteasome inhibitor MG132 (20 μm, 1 h), radiolabeled, and permeabilized. Cells were then incubated in the presence of ARS for either 0 or 60 min at 37 °C. A portion of the sample (T) was directly analyzed, whereas the other was fractionated into supernatant (S) and membrane pellet (P). The extracts were subjected to immunoprecipitation with αHC. HC+CHO, glycosylated HC; HC-CHO, deglycosylated HC. c, MG132-treated US2 cells were radiolabeled and permeabilized. The membrane pellet was washed with a buffer containing the indicated concentrations of salt before being incubated with CLC or a buffer at 37 °C. Samples taken at the indicated time points were directly analyzed by immunoprecipitation with αHC. d, the membrane fraction from radiolabeled, MG132-treated US2 cells were salt treated and incubated with CLC either in the absence or presence of ARS before being subjected to immunoprecipitation with αHC. The image is a representative gel from three independent experiments.