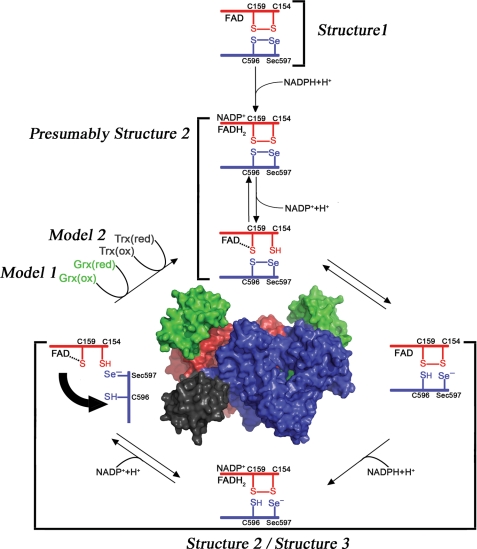
FIGURE 1.
Electron transfer in and out of the TR domain of SmTGR. The reaction scheme has been drawn following the experimental evidence reported in this work and elsewhere (18). When NADPH binds to the oxidized state of the enzyme (Structure 1), electrons are transferred from the nicotinamide ring to the FAD; reduced FADH2 donates electrons to the Cys154–Cys159 couple (in red) that is close to the isoalloxazine ring of the cofactor (as observed in Structure 2), and finally electrons reach the Cys596–Sec597 redox couple on the C-terminal segment of the other subunit (in blue, as observed in Structure 3). The C terminus acts as a flexible arm, which might donate electrons either internally to the Cys28–Cys31 redox active couple on the oxidized Grx domain (in green) or externally to various oxidized substrates such as SmTrx (in dark gray).