FIGURE 2.
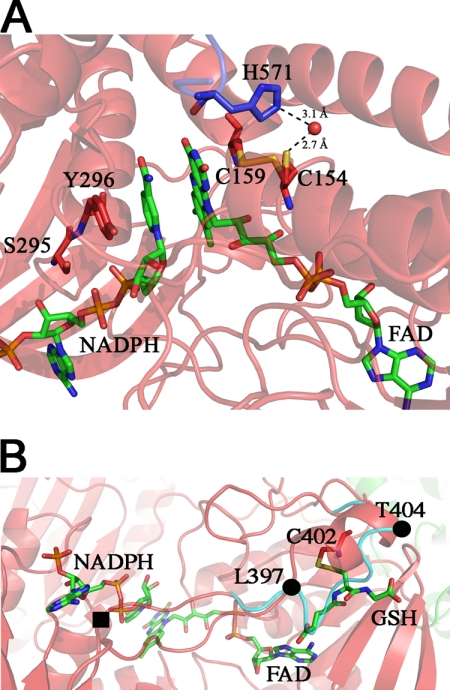
Structure 2, NADPH-binding site in SmTGRfl. Panel A, zoom in the FAD active site. The NADPH and FAD are shown as green sticks; Tyr296, Ser295, Cys154, and Cys159 are shown as red sticks, and His571 of the partner subunit is shown as blue sticks. Upon NADPH binding, the loop 295–297 changes conformation re-orienting the side chains of Ser295 and Tyr296 with respect to Structure 1 to make room for the reductant. The Cys159–Cys154 couple is partially reduced, and accordingly, Cys154 is found in a double conformation. In the reduced conformation (50% occupancy), the sulfur of Cys154 points toward the solvent and is in contact with a water molecule (2.7 Å, shown as red ball), which is kept in place by His571. Panel B, GSH-binding site on TR domain of Structure 2. The GSH (green sticks) is found in a position that in other TGR structures is usually occupied by the loop 397–404 (cyan ribbon). The ligand is found in a pocket formed by one α-helix (Leu397–Thr404), a β-strand (Lys227–Leu230), and the nucleotide moiety of the FAD. GSH makes a mixed disulfide with Cys402 and forces the loop 397–404 to adopt a α-helical secondary structure (red ribbon) by turning around two pivot residues Leu397 and Thr404 (shown as full black circles). One of the gates of the NADPH-binding site (full black square) is four residues upstream of Leu397.