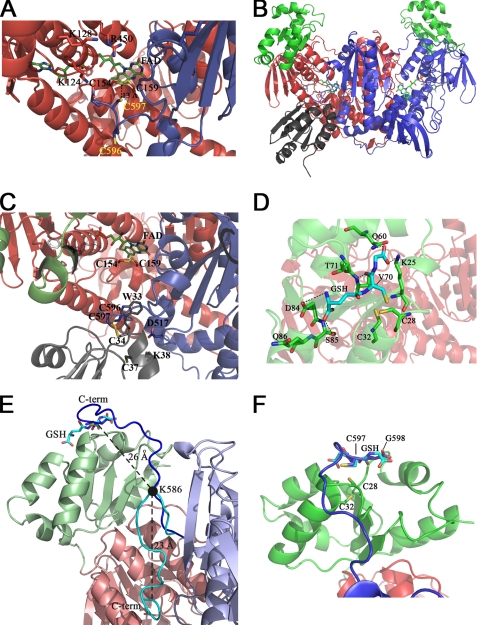
FIGURE 3.
Structure 3 and 4 and Models 1 and 2. The three-dimensional structure of the C-terminal region of SmTGRfl and the computed model of SmTGRfl-SmTrx complex are shown. The GSH-binding site of the Grx domain and a model of the C terminus pointing in its direction are shown. Panel A, Structure 3, ribbon representation of the C-terminal region of SmTGRfl. The two catalytic Cys belonging to the C-terminal arm (Cys596 and Cys597), shown as blue sticks, are both reduced; Cys597 is located about 13 Å from the Cys154–Cys159 couple (see text). The positively charged residues Lys124, Lys128, and Arg450 of one subunit involved in the stabilization of the C-terminal arm of the partner subunit are shown as red sticks. Panel B, Model 2, relative location of SmTrx and SmTGR in the modeled complex is shown. Components are as follows: SmTrx (gray); subunit A (red); subunit B of SmTGR (blue); Grx domains (green). Panel C, SmTrx-binding site on SmTGR in Model 2. The magnification allows us to visualize the relative topology of the redox sites (FAD, Cys154/Cys159, C terminus (Cys596/Cys597), and SmTrx (Cys34–Cys37)) involved in the electron transfer chain. The redox sites of both enzymes and the main side chains involved in the contact between SmTrx and SmTGR are shown as sticks. Panel D, GSH-binding site on the Grx domain. GSH (cyan sticks) is in a pocket above the redox site of the Grx domain (Cys28–Cys32, green sticks). Polar contacts are shown by dotted lines. The γ-glutamyl moiety of GSH interacts with Asp84, Ser85, and Gln86 (green sticks). The GSH sulfur points toward Cys28, and its position is stabilized by contacts with Thr70 and Val71. The carboxylate of the glutamic acid of GSH is H-bonded to Gln60 and Lys25. Panel E, superposition between Structure 3 and Model 1. The ribbon representation depicts the movement of the C-terminal arm. A rotation on the pivot residue Lys586 (visualized as a full black circle) brings the C terminus from the position found in Structure 3 (cyan) to the Grx domain (from Model 1, blue). The superposition between the last two residues of the C terminus (Cys597–Gly598, blue sticks) and the analogue residues of GSH (cyan sticks, as bound in Structure 4) is also shown. The distances between Cα of Lys586 and Cα of Cys597 and that between the former and the Cα of the GSH Cys are shown in the figure. Panel F, zoom of the superposition between Cys597–Gly598 (blue sticks) of the C-terminal arm and the GSH (cyan sticks) onto the Grx redox site (green sticks).