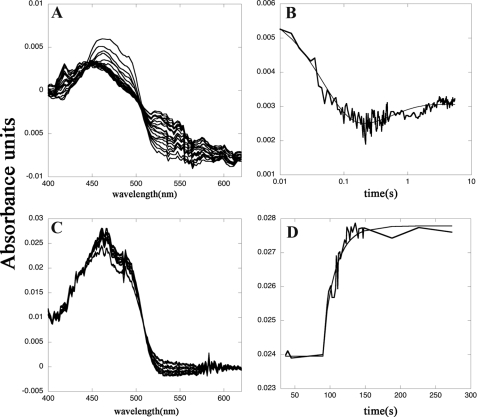
FIGURE 4.
Reductive and oxidative half-reactions of SmTGRfl, spectrophotometric experiments. Panel A, spectra of 2.5 μm SmTGRfl reduced with 5 μm NADPH recorded in a stopped-flow apparatus. The decrease of the FAD peak at 460 nm and the increase of the absorbance around 550 nm due to the formation of the charge transfer complex are shown. Spectra were recorded every 5 ms from 5 to 500 ms and then every 50 ms up to 5500 ms. Panel B, time course recorded at 460 nm relative to the experiment reported in panel A fitted with a double exponential. The first process is assigned to the reduction of the FAD by NADPH (k = 4 × 106 m−1 s−1) to form the two-electron reduced species EH2; the second process is the perturbation of the cofactor spectrum due to the formation of EH4 (k = 1 × 106 m−1 s−1). These two processes are compatible with electrons transferred from NADPH to Structure 1 and from the reduced Cys154–Cys159 couple (as found in Structure 2) to the C-terminal redox center, allowing the formation of its reduced form as found in Structure 3. Panel C, spectra of reduced SmTGRfl (3.0 μm) oxidized with SmTrx (7 μm) were recorded in a spectrophotometer apparatus. The decrease of absorbance of the charge transfer complex around 550 nm and the increase of the FAD peak at 460 nm is shown. Panel D, time course at 460 nm relative to the experiment reported in panel C fitted to a single exponential. The second-order process is assigned to the oxidation of the FADH2 of SmTGRfl by SmTrx (k = 0.8 × 104 m−1 s−1). This step is compatible with the electron transfer from the reduced C terminus, as found in Structure 3, to SmTrx (see Fig. 1).