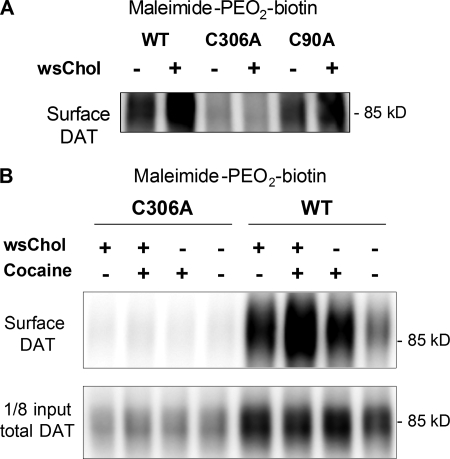
FIGURE 4.
Sulfhydryl side chain of Cys-306 in DAT exhibits conformational sensitivity to membrane cholesterol content. A, representative blot (n = 3) from HEK293 cells transiently transfected with WT, C306A, and C90A DAT. Cells were treated with 0.1 mg/ml wsChol for 15 min at 37 °C and incubated with 1 mg/ml maleimide-PEO2-biotin for 0.5 h at 4 °C. The enhanced biotinylation of sulfhydryl groups was not seen with the C306A mutant. B, HEK293 cells stably expressing wild-type and C306A DAT were probed with maleimide-PEO2-biotin following wsChol treatment (0.05 mg/ml; 15 min at 37 °C). Where indicated, cocaine (1 mm) was included in the biotinylation buffer. Compared with the WT DAT, almost no biotinylated signals were detected with the C306A DAT. Despite its low expression level, C306A was expressed on the cell surface and exhibited functional substrate uptake and ligand binding (see Fig. 6A).