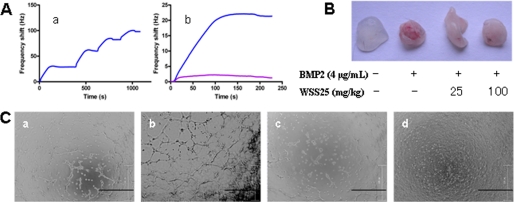
FIGURE 6.
Interaction of WSS25 with BMP2 and its effects on BMP2-induced angiogenesis. A, WSS25 strongly bound to BMP2 by QCM analysis. The WSS25 biosensor surface was prepared for measuring carbohydrate-protein interactions, where the frequency shift produced from biotinylated WSS25 binding to the streptavidin surface is shown in (panel a). The WSS25-BMP2 interaction was tested by injecting BMP2 (50 μg/ml, 50 μl) in running buffer onto the WSS25 biosensor surface, and the frequency response is displayed in panel b (blue curve). The large shift produced indicated that WSS25 bound to BMP2 strongly. As a control, the frequency responses by the BMP2 interaction with the streptavidin surface were measured, yielding a small response in panel b (pink curve). These results indicate the BMP2-WSS25 interaction was specific. B, WSS25 inhibited BMP2-induced angiogenesis in the Matrigel plug assay. Matrigel (500 μl) with or without BMP2 (4 μg/ml) was subcutaneously injected into the ventral region of C57/BL6 mice. WSS25 (25 mg/kg or 100 mg/kg body weight) was administered to the mice every other day from the second day after the Matrigel was plugged into the mice. Normal saline was used as the control. C, WSS25 and Noggin inhibited BMP2-induced tube formation of HMEC-1 cells on Matrigel. Growth factor reduced Matrigel (50 μl/well) was added to a 96-well plate to be solidified in 37 °C for 30 min. HMEC-1 cells (3 × 104 cells in 98 μl MCDB131 medium supplemented with 0.1% FBS per well) were seeded into the 96-well plate after the Matrigel solidification. BMP2 (200 ng/ml) was added together with the cells only (panel b), or in the presence of Noggin (1 μg/ml) (panel c) or WSS25 (25 μg/ml) (panel d). Photos were taken after incubation for 24 h at 37 °C.