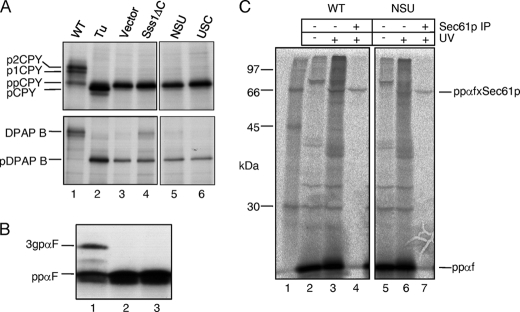
FIGURE 5.
Protein translocation phenotypes of Sss1p domain mutants. A, FKY198 cells containing control or Sss1p mutant plasmids were grown in glucose medium for 6 h prior to 35S labeling for 5 min. Whole cell extracts were immunoprecipitated with ppCPY- or DPAP B-specific antiserum, and the precipitates were resolved by 10 and 7.5% SDS-PAGE, respectively (panels edited from the same gels). Upon ER translocation, ppCPY is signal-cleaved and modified by N-linked glycan addition in the ER (p1CPY) and the Golgi (p2CPY). The type II membrane protein, DPAP B, acquires N-linked glycans upon correct ER membrane integration. Tunicamycin (Tu) treatment yielded signal-cleaved, but unglycosylated pro-CPY (lane 2) and the unglycosylated pre-form of DPAP B (pDPAP B). B, post-translational translocation in vitro. Membranes from FKY198 cells containing Sss1p (lane 1), vector (lane 2), or the NSUp-expressing plasmids (lane 3) after 6 h in glucose were incubated with a 35S/TDBA-labeled K5/K14-ppαf substrate, with visualization after 12.5% SDS-PAGE. The 3gpαf translocated and glycosylated form is indicated. C, analysis of precursor targeting to Sec61p. Membranes prepared from FKY198 cells containing Sss1p (WT) or the NSUp-expressing plasmids after 6 h of growth in glucose medium were incubated with the 35S/TDBA-labeled K5/K14-ppαf after ATP-depletion. Two-thirds of each reaction was UV-irradiated, and half of this was precipitated with Sec61p-specific antiserum. The reaction products were visualized after 12.5% SDS-PAGE, and cross-links between ppαf and Sec61p are indicated (ppαfxSec61p). Lane 1 contains radiolabeled protein standards. The data in A and B are representative of two independent experiments, and the data in C are representative of three independent experiments.