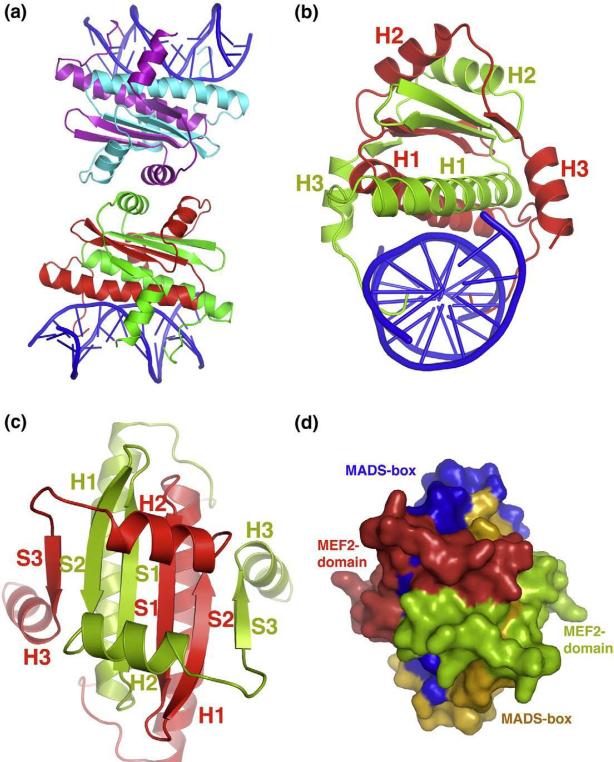
Fig. 1.
Overall structure and packing of MEF2 dimer bound to DNA. (a) Asymmetric unit containing two independent MEF2–DNA complexes stacking head-to-head is shown in cartoon diagram. Two monomers in one complex are colored in green and red, respectively, while in the other complex they are colored in cyan and violet, respectively. DNA is colored in blue throughout the illustration. (b) MEF2–DNA complex forming an intertwined dimer is shown in cartoon diagram along the DNA axis. One monomer is colored in red and the other in green. All three α helices for both the monomers are labeled in corresponding colors. (c) Top view of the same complex shown in (b). Here, all three β sheets along with the three α helices of both the monomers are labeled in corresponding colors. DNA is omitted in this view for clarity. (d) Surface representation with the same orientation as shown in (c). Here, MADS-box and MEF2 domain are colored in orange and red in one monomer, respectively. Corresponding colors in the other monomer are blue and green, respectively. This representation shows that the MADS-box and MEF2 domain of the two monomers form an intimately folded domain.