Table 1.
DDQ-catalyzed oxidative cyclization reactions.a
| entry | substrate | product | time (h) | yield (%)b |
|---|---|---|---|---|
| 1 |

|
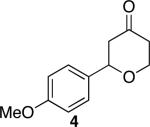
|
41 | 76 |
| 2 |

|
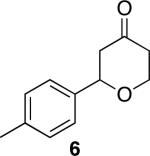
|
44 | 83 |
| 3 |
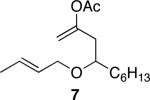
|
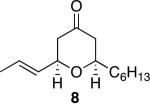
|
40 | 92 |
| 4c |
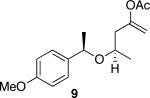
|

|
48 | 75 |
| 5 |
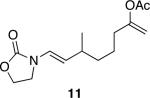
|
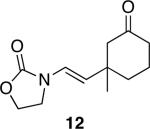
|
36 | 68 |
| 6 |
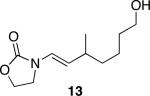
|
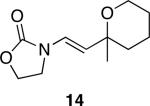
|
24 | 87 |
| 7 |
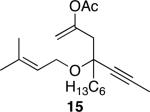
|

|
40 | 70 |
| 8 |
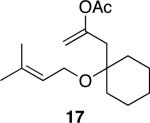
|

|
30 | 81 |
| 9 |

|
– | – | – |
Representative procedure: DDQ was added in three equal portions over the course of the reaction (0.15 equiv total) to a suspension of the substrate, 2,6-dichloropyridine (2 equiv), and activated MnO2 (6 equiv) in CH3NO2 (0.1 M). The reaction stirred at room temperature for the indicated period of time.
Yields refer to isolated, purified products.
Reaction required 0.2 equiv DDQ.
