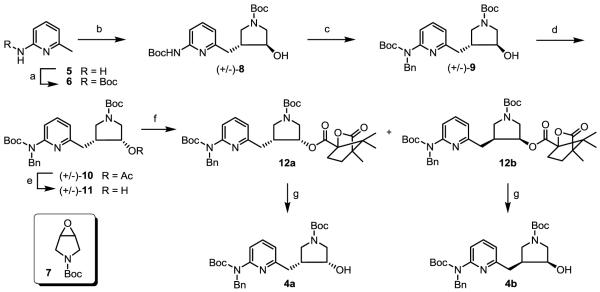
Scheme 1.
Synthesis of 4a and 4b.
a Reagents and conditions: (a) (Boc)2O, TEA, t-BuOH, 50 °C, 24 h, 90%; (b) (i) n-BuLi (2 equiv.), −78 °C to r.t., 30 min, (ii) 7, −78 °C to r.t., 3 h, 55%; (c) NaH, 0 °C to r.t., 10 min, benzyl bromide, r.t., 8 h, 92%; (d) PPh3, DIAD, acetic acid, r.t., 12 h, 95%; (e) NaOH (2N)/MeOH, 50 °C, 4 h, 90%; (f) (1S)-(−)-camphanic chloride, TEA, DMAP, r.t. 4 h, 49% for each diastereomer; (g) Na2CO3, H2O/MeOH, r.t., 3 h, 99%.