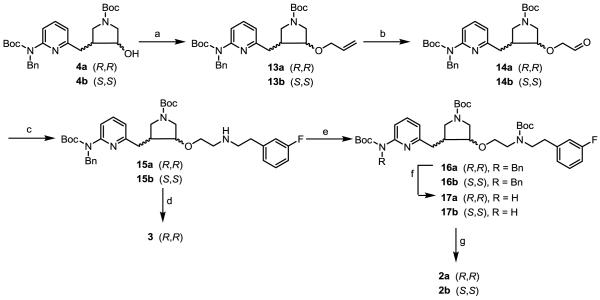
Scheme 2.
Syntheses of 2a, 2b, and 3.
a Reagents and conditions: (a) NaH, allyl bromide, 0 °C to r.t. 30 min, 99%; (b) (i) O3, −78 °C, (ii) Zn, acetic acid, −78 °C to r.t., 70-75%; (c) 2-(3-fluorophenyl)ethanamine, NaHB(OAc)3, r.t., 3 h, 52-55%; (d) Pd(OH)2/C, H2, 2:1 EtOH/HCl (12 N), r.t., 575 psi, 40 h, 100%; (e) (Boc)2O, Et3N, MeOH, r.t., 0.5 h, 99%; (f) Pd(OH)2/C, H2, 60 °C, 24 h, 35-50%; (g) 6 N HCl/MeOH (2:1), r.t., 4 h, 80-85%.