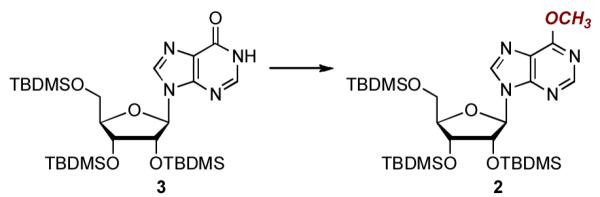
Table 2.
Evaluation of Conditions for a One-Pot Conversion of Trisilyl Inosine 3 to the O6-Methyl Ether 2
 | |||
|---|---|---|---|
| entry | conditionsa | timeb | % yieldc |
| 1 | DBU (4 molar equiv), BOP (2 molar equiv), MeOH (20 molar equiv), THF, rt |
24 h | --d |
| 2 | DBU (4 molar equiv), BOP (2 molar equiv), THF, rt Then evaporate solvent and add MeOH (20 molar equiv), rt |
step 1: 2 h step 2: 24 h |
--e |
| 3 | DBU (2 molar equiv), BOP (2 molar equiv), THF, rt Then add DBU (2 molar equiv) and MeOH (20 molar equiv), rt |
step1: 2 h step 2: 8 h |
74 |
| 4 | DBU (2 molar equiv), BOP (2 molar equiv), THF, rt Then evaporate solvent and add DBU (2 molar equiv) and MeOH (20 molar equiv), rt |
step 1: 2 h step 2: 4 h |
76 |
| 5 | Cs2CO3 (4 molar equiv), BOP (2 molar equiv), MeOH (20 molar equiv), THF, rt |
48 h | --d |
| 6 | Cs2CO3 (4 molar equiv), BOP (2 molar equiv), THF, rt Then add MeOH (20 molar equiv), rt |
step 1: 20 min step 2: 24 h |
--e |
| 7 | DBU (2 molar equiv), BOP (2 molar equiv), THF, rt Then evaporate solvent and add Cs2CO3 (2 molar equiv) and MeOH (20 molar equiv), rt |
step 1: 2 h step 2: 1 h |
77 |
| 8 | Cs2CO3 (2 molar equiv), BOP (2 molar equiv), THF, rt Then add Cs2CO3 (2 molar equiv) and MeOH (20 molar equiv), rt |
step 1: 10 min step 2: 2 h |
94 |
| 9 | Cs2CO3 (2 molar equiv), BOP (2 molar equiv), THF, rt Then evaporate solvent and add Cs2CO3 (2 molar equiv) and MeOH (20 molar equiv), rt |
step 1: 10 min step 2: 10 min |
94 |
Reactions were conducted using 0.16 mmol of 3 in THF (2 mL).
Reactions were monitored by TLC.
Yields reported are of isolated and purified product.
Mixture of products.
Formation of the O6-(benzotriazol-1-yl) derivative 1 was complete as observed by TLC.
