Fig. 1.
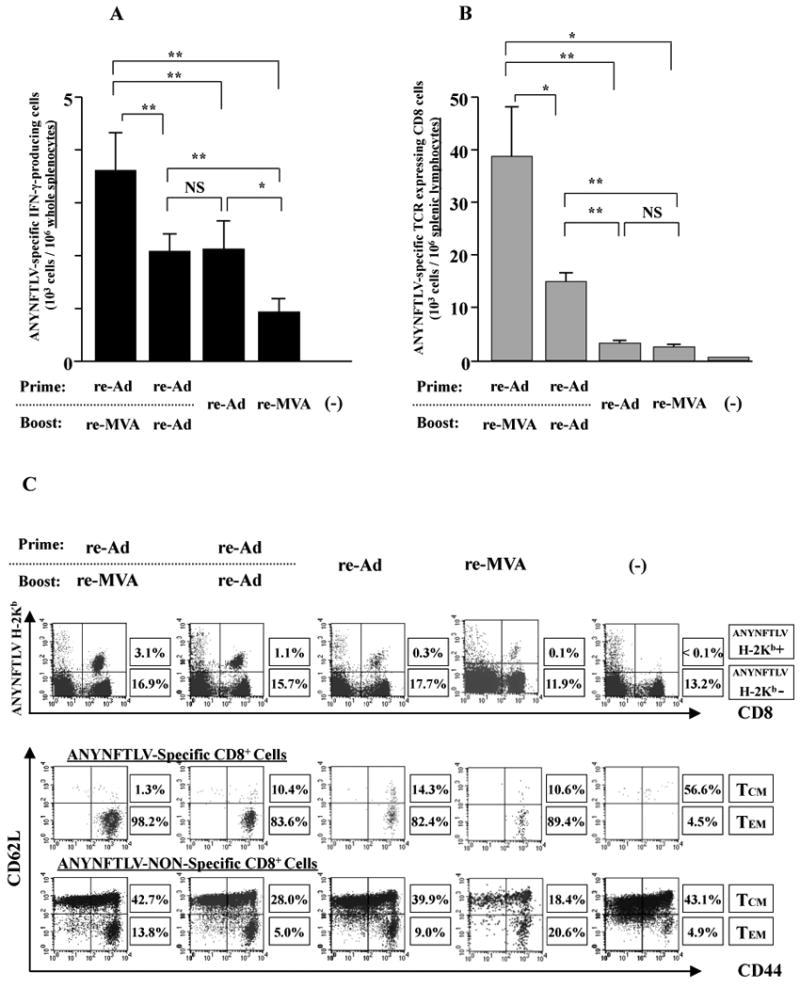
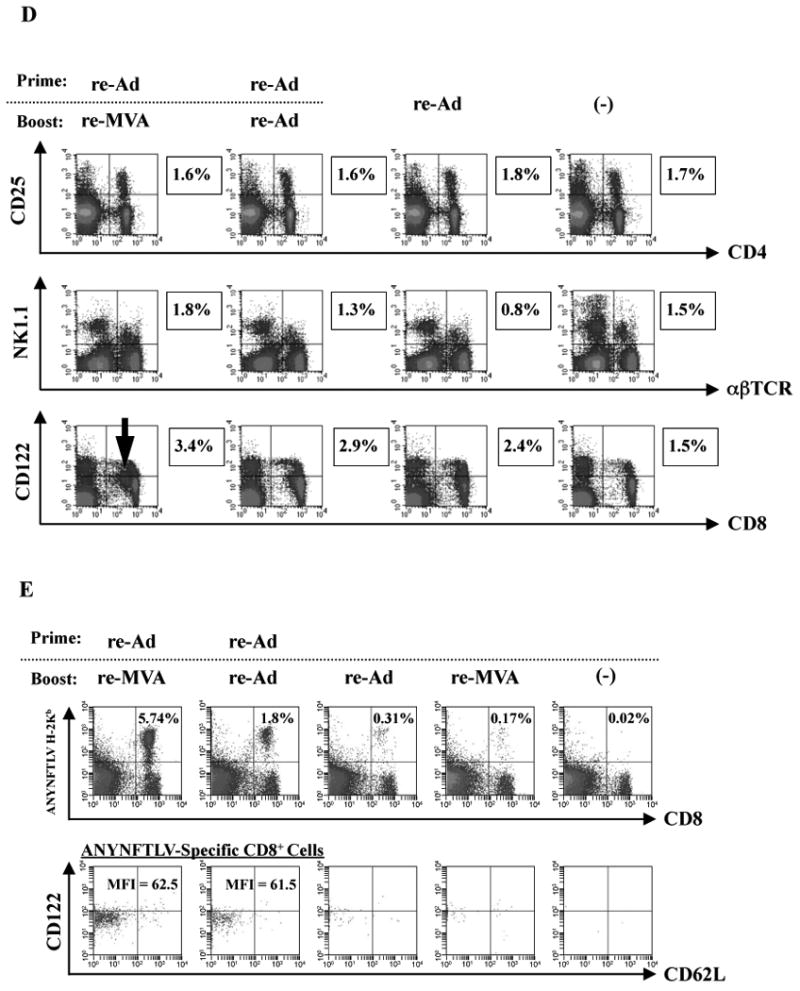
Phenotypic features of ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells induced by the use of recombinant adenoma virus (re-Ad) and recombinant vaccinia virus (re-MVA). B6 mice were first primed with 5 × 107 plaque forming units (PFU) of re-Ad and then were given a booster immunization 14 days later with 5 × 107 PFU of either re-Ad or re-MVA. The single re-Ad immunized and non-immunized mice were included as controls. The mice were sacrificed 12 days after the last immunization and spleens were removed. The freshly-isolated splenocytes were subjected to immunological assays. (A) The splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells per 106 splenocytes was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three to nine mice in each group. **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”. (B) The splenocytes were subjected to flow cytometory (FCM) analyses for detecting Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The number of ANYNFTLV+, CD8+ T cells per 106 splenic lymphocytes was calculated. Data represent the mean ± standard deviation of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. (C) The representative data of the FACS analyses for detecting Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. (D) The representative data of FACS analyses for detection of “regulatory” cell populations. After collecting immune splenocytes, the FITC-conjugated anti-TCRb chain mAb, the PE-conjugated anti-NK1.1 mAb, the PE-conjugated anti-CD122 mAb, the PerCP-Cy5.5-conjugated anti-CD4 mAb and the PerCP-Cy5.5-conjugated anti-CD8 mAb were used to stain immune cells for the identification of CD4+CD25+ T cells, natural killier (NK) T cells and CD8+CD122+ T cells. (E) The representative data of FACS analyses for detection of a Kb/ANYNFTLV pentamer-reactive, CD122dull cell population. The TCR was stained with the PE-conjugated Kb/ANYNFTLV pentamer, the PerCP-Cy5.5-conjugated anti-CD8 mAb, the APC-conjugated anti-CD122 mAb and the FITC-conjugated anti-CD62L mAb. The mean fluorescence intensity (MFI) of CD122 staining was calculated by gating the ANYNFTLV+, CD8+ cells. The single re-MVA immunized mice were also included for this experiment. All of the experiments were repeated at least twice for the confirmation of reproducibility. The use of control Ad and control MVA which are not expressing the epitope did not induce the ANYNFTLV-specific immune responses (data not shown).
