Fig. 4.
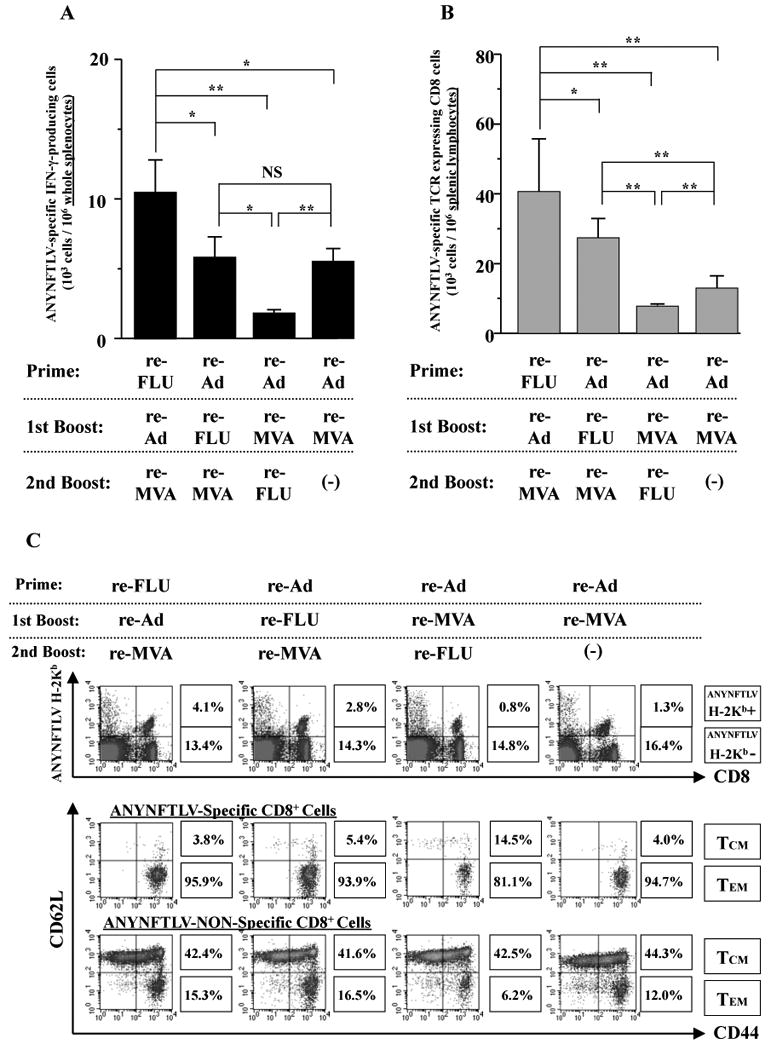
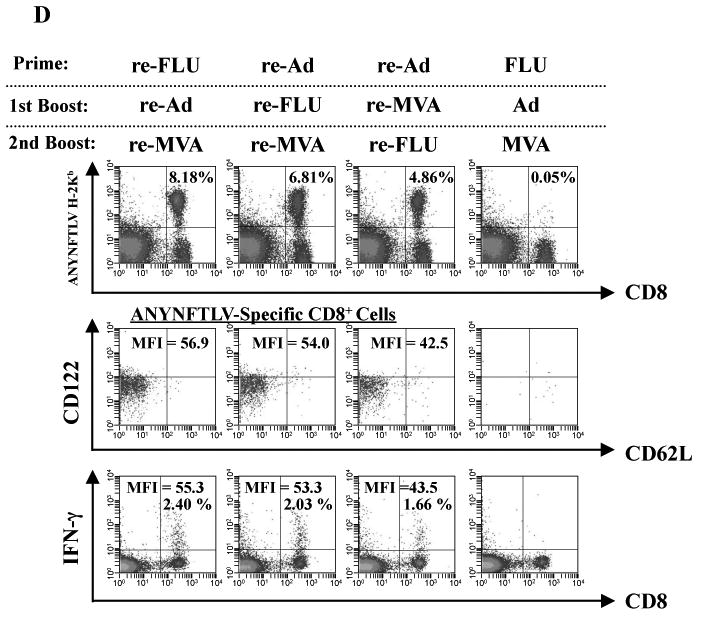
Phenotypical and immunological features of ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells induced by triple virus vector immunization. B6 mice were first primed with 1 × 104 plaque forming units (PFU) of recombinant influenza virus (re-FLU) or 5 × 107 PFU of recombinant adenovirus (re-Ad), followed by booster immunizations 14 days later with 1 × 104 PFU of re-FLU, 5 × 107 PFU of re-Ad or 5 × 107 PFU of recombinant vaccinia virus (re-MVA). They were further given second booster immunizations 14 days later with 1 × 104 PFU of re-FLU, or 5 × 107 PFU of re-MVA. (A) The mice were sacrificed 12 days after the last immunization, and spleens were removed. The freshly-isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells × 106 was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”. (B) The same immune splenocytes in (A) were subjected to the flow cytometory (FCM) analyses for detection of Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The number of ANYNFTLV+, CD8+ T cells per 106 splenic lymphocytes was calculated. Data represent the mean ± S.D. of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. (C) The representative data of the FACS analyses for detection of Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. (D) The mice were sacrificed 14 days after the last immunization, and spleens were removed. The representative data of the FACS analyses showed the detection of Kb/ANYNFTLV pentamer-reactive, CD122dull cell population and the IFN-γ+, CD8+ T cell population. The TCRs were stained with the PE-conjugated Kb/ANYNFTLV pentamer, the PerCP-Cy5.5-conjugated anti-CD8 mAb, the APC-conjugated anti-CD122 mAb and the FITC-conjugated anti-CD62L mAb. The MFI of CD122 staining was calculated by gating the ANYNFTLV+, CD8+ cells. The same immune splenocytes were also subjected to the FACS analyses for the detection of intracellular IFN-γ+ cells in response to the ANYNFTLV-pulsed EL-4 cells. The cell culture condition for the assays was described in the Materials and methods. The number of IFN-γ+ cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ+ cells that appeared against peptide-pulsed EL-4. The mean fluorescence intensity (MFI) of ANYNFTLV-responding IFN-γ+ cells was calibrated by gating cells which were both IFN-γ-positive and CD8-positive. The use of control Ad, control MVA and control FLU which do not express the epitope did not induce the ANYNFTLV-specific immune responses (data not shown). All the experiments were repeated at least twice for the confirmation of reproducibility.
