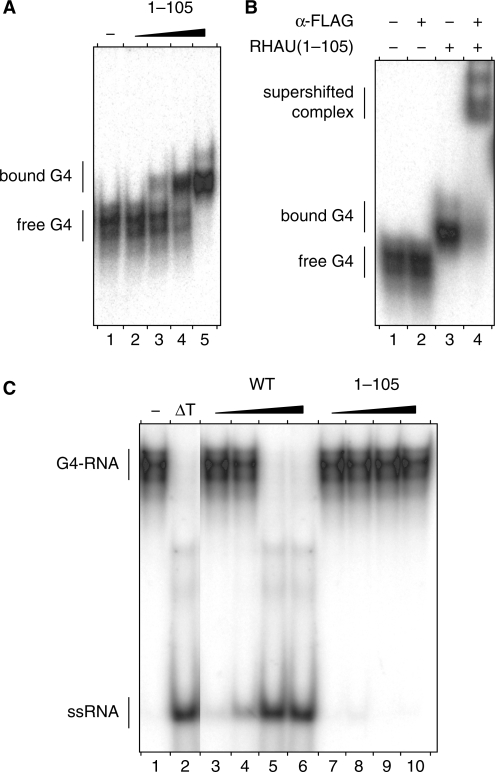
Figure 3.
G4-RNA binding and resolving activities of the N-terminal domain of RHAU. (A) Gel mobility shift assay for G4-RNA binding: radio-labelled tetramolecular rAGA at a concentration of 100 pM was incubated without protein (−) or with increasing amounts (30, 100, 300 and 1000 nM) of RHAU(1–105) in the absence of ATP. The reaction mixtures were analysed by native PAGE. An autoradiogram of the gel is shown. (B) Super shift of G4-RNA-binding complex by a specific antibody: radio-labelled tetramolecular rAGA at a concentration of 100 pM was incubated with RHAU(1–105) at 300 nM in the presence or absence of anti-FLAG antibodies (2.5 μg per lane), as indicated. The reaction mixtures were electrophoresed on a native gel. An autoradiogram of the gel is shown. The positions of the free tetramolecular RNA substrate, the protein–RNA complex and the super-shifted complex are indicated on the left. (C) G4-RNA unwinding assay: radio-labelled tetramolecular rAGA at a concentration of 4 nM was incubated in the presence of ATP without protein (−) or with increasing amounts (1, 3, 10 and 30 nM) of WT RHAU or ΔGly–RSM mutant (30, 100, 300 and 1000 nM). The reaction products were resolved by native PAGE after disrupting of RNA–protein interactions with SDS. An autoradiogram of the gel is shown. An aliquot of the tetraplex substrate that was heat-denatured (95°C, 5 min) and then quenched (ΔT) serves as a marker for the position of ssRNA.