Abstract
Background
Previous experimental and laboratory studies have implicated antibodies against Hu proteins (anti-Hu) as a potential marker for small cell lung cancer (SCLC); there are no estimates of the association between anti-Hu and SCLC using a population-based design.
Methods
We used stored plasma specimens to evaluate anti-Hu reactivity in relationship to small cell lung cancer in a population-based case-control study. Using Western Blot analysis, we measured anti-Hu reactivity against recombinant Hu family member, HuD, in plasma samples from forty-one (41) SCLC cases and seventy-nine (79) controls individually matched for age, race, sex, and smoking status (never, past, current). We analyzed the association between anti-Hu reactivity and SCLC using conditional logistic regression.
Results
Anti-Hu reactivity was associated with SCLC, both before and after adjustment for amount of smoking. We observed a smoking-adjusted odds ratio of 3.2 (95 % confidence interval from 0.98 to 13.4) comparing subjects above 1800 units (the lower limit of the second tertile of the distribution among antibody positive controls) to subjects with lower reactivity. We also found suggestive evidence in follow-up of our cases that anti-Hu above 1800 units was related to longer-term survival from SCLC. The present research is the first report of anti-Hu reactivity and SCLC in a population-based study.
Conclusions
Given the suggestive evidence in this study, prospective analyses to examine whether anti-Hu reactivity might predict risk of developing SCLC, or whether anti-Hu reactivity could serve as an early marker for SCLC, may be warranted.
Keywords: carcinoma, small cell, Hu paraneoplastic encephalomyelitis antigens, HuD antigen, autoantibodies, case-control studies, survival
Introduction
Establishment of association with the disease in question is an important first step in biomarker identification. Biomarkers should ideally reflect and/or predict the disease with high specificity and sensitivity [1]. Results from small clinical samples can provide important first clues to potential biomarker-disease associations that can later be examined in larger study designs.
Lung cancer is the leading cause of cancer death in the United States and Western Europe. Small cell lung cancer (SCLC), showing properties of primitive neuroendocrine cells [2], accounts for up to 13% of all newly diagnosed lung cancers [3] and is strongly associated with cigarette smoking [4–8]. Initially, SCLC patients respond well to chemotherapy, however, relapses are inevitable and are usually resistant to cytotoxic treatment; only 10% of all SCLC patients have significant long-term survival [9].
Paraneoplastic encephalomyelitis/sensory neuronopathy (PEM/SN) is one of a number of rare paraneoplastic autoimmune diseases associated with SCLC. PEM/SN is characterized by dementia, sensory loss, and other neurological disabilities [10]. SCLC patients with PEM/SN have high titers of antibodies that react against neuronal nuclear proteins of 35–40 kDa known as Hu proteins [11;12]. Hu proteins are a family of four RNA-binding proteins, three of which--HuB/Hel-N1, HuC, and HuD-- are normally restricted to the nervous system, although HuB/Hel-N1 has also been detected in the testes and ovaries [13]. Hu proteins are homologous to the embryonic lethal abnormal visual (elav) protein in Drosophila and play a role in neuron-specific RNA processing and neural development [12;14–16]. In SCLC, Hu antigens are abnormally expressed in the tumor, characterizing them as onconeural antigens. Generation of anti-Hu autoantibodies is thought to be part of an immune response which cross-reacts with the healthy nervous system, resulting in PEM/SN [17]. The neurological disorder, rather than the cancer, is usually the cause of death in SCLC patients with PEM/SN [18].
All SCLC tumors, whether from patients with or without PEM/SN, express neuronal Hu proteins [12;19;20]. Dalmau et al. and Graus et al. found that ~16% of SCLC patients without paraneoplastic neurological autoimmune syndromes have detectable titers of anti-Hu antibody in their serum, albeit at much lower levels than PEM/SN patients [10;21]; additional studies using similar techniques conducted by Verschuuren et al and Monstad et al found anti-Hu reactivity in 17% and 25.5% of SCLC cases, respectively [22;23]. No studies have yet evaluated anti-Hu antibodies among healthy subjects from population-based studies, and overall population prevalence is unknown.
Although anti-Hu antibodies are also found in a fraction of neuroblastoma patients, they are rarely present in other cancers. Thus, the presence of anti-Hu antibodies in patient serum may serve as a marker for SCLC, and as a model for antibody-based early cancer detection, and may function as a prognostic indicator. Other paraneoplastic diseases, such as Lambert-Eaton myasthenic syndrome (LEMS) [24;25], limbic encephalomyelitis (LE) [26;27], opsoclonus myoclonus syndrome [28], and cancer-associated retinopathy [29;30] are also associated with SCLC, indicating that SCLC patients may express additional cancer-specific antibodies against neuronal proteins [31]. In theory, if a large enough panel of SCLC-associated antigens could be identified, these antigens could carry potential value for early detection of this disease. Because SCLC is so rapidly metastatic, it has been argued that early detection (and ensuing intervention) of SCLC is not feasible. However, because the process by which SCLC develops is unknown, the possibility that such antibodies might be detectable in SCLC at a stage prior to rapid disease progression cannot be excluded.
To determine whether anti-Hu antibodies are associated with increased risk of developing SCLC, we studied the anti-Hu response in a population-based case-control study that included healthy smoking and non-smoking controls and small cell lung cancer cases. To this end, we analyzed anti-Hu antibody levels in 41 SCLC patient plasma samples and 79 matched population controls. We additionally studied the association between anti-Hu antibodies and survival time among the cases.
Materials and Methods
Plasma Samples and Study Population
Plasma specimens were previously collected from a population-based case-control study of African Americans and Caucasians from Los Angeles County, California, that was conducted during the early to mid-1990s [32]. Participation in the study included provision of a blood sample and completion of an in-person interview on risk factors for lung cancer, including smoking history. Plasma had been stored at −80°C since the original blood collection. Among the 342 lung cancer cases that participated in the original study, 46 were diagnosed with SCLC. Stored plasma was available for 41 SCLC cases.
Because we wanted to examine whether smoking was associated with anti-Hu reactivity among the controls, we selected two control groups (smokers and never smokers). Controls were individually matched to SCLC cases on age (within 5-year age group), race, and gender. The first set of controls consisted of current smokers or former smokers, matched to the smoking status of the case. The second control group consisted of never smokers. If multiple smoking and non-smoking controls matched to a SCLC case, one control of each was chosen at random. We analyzed plasma from 120 subjects including 79 healthy controls (40 smokers and 39 non-smokers) and 41 SCLC cases.
Written informed consent was obtained from each study participant. The University of Southern California institutional review board approved the use of human subjects in accord with an assurance filed with and approved by the US Department of Health and Human Services.
Purification of HuD recombinant protein
Anti-Hu sera cross-react with all three neuronal Hu antigens, which are highly conserved [20]. While all SCLCs express neuronal Hu antigens, HuD was reported to be most commonly expressed [20]. Thus we determined anti-HuD reactivity in our samples using recombinant HuD protein, prepared as described [33], with the following modifications. The sonication buffer consisted of 20mM HEPES (pH 7.4), 150mM NaCl, and 0.5% Triton X-100. Proteins were eluted from Ni2+ beads using sonication buffer containing 10% glycerol and 50–250mM imidazole. The protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA) and verified on Coomassie blue-stained gels by running dilutions of the purified protein along a similar molecular weight standard of known concentration.
Western Blotting
Protein (1ug in 20ul total volume) was resolved on 10% sodium dodecyl sulfate (SDS) gels and transferred to nitrocellulose filters as described in Towbin et al. with the following modifications [34]. Transfer was performed for 45 minutes at 100 V while chilling buffer with a cold pack. The filters were blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline, Tween-20 (TBST: 10 mM Tris/HCL pH 8.0 150mM NaCl, 0.05% Tween-20) overnight at room temperature. The following day, blots were incubated with 1:1000 dilution of plasma in 3% BSA/TBST for 2 hours at room temperature followed by three washes for 10 minutes with 0.3% BSA/TBST at room temperature. Secondary antibody, anti-human horse-radish peroxidase (Sigma Product No. A-0170), was diluted 1:2000 in TBS and added for 1 hour. After washing five times for 6 minutes in TBS, the blot was submerged in SuperSignal® West Substrate Working Solution, prepared according to the manufacturer’s instructions (Pierce, Product No. 34080). The blots were imaged using Bio-Rad Fluor-S™ MultiImager, and images were taken every 20 seconds during a 5 minute exposure time
Determination of Anti-Hu Antibody Reactivity
Previously, it was shown that use of recombinant HuD in a Western blot analysis is the most sensitive and specific method for the detection of anti-Hu activity [10;35]. Older analyses in which neuronal lysates were used run the risk of showing reactivity to unknown proteins of similar molecular weight. Even when using affinity purified HuD from bacterial lysates, occasionally background bands of the wrong size are observed. Caution must therefore be used when determining anti-Hu reactivity. In our analysis, only plasma samples showing a band of the correct size (~40 kDa for the tagged recombinant HuD) were scored as reactive against Hu proteins.
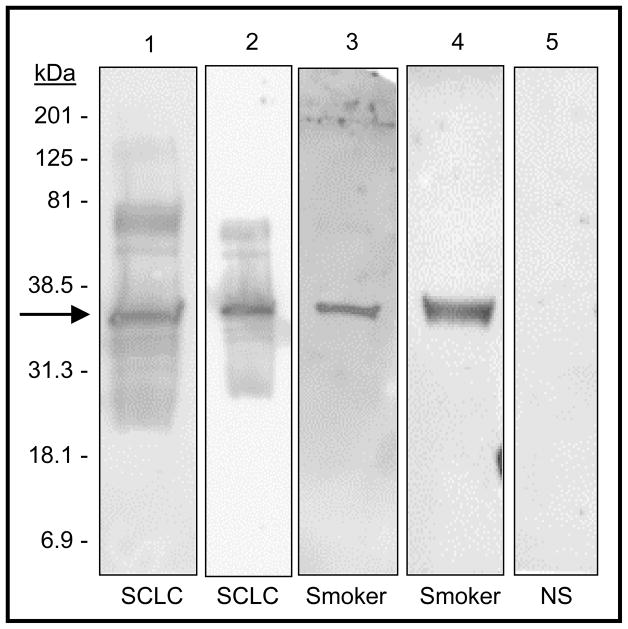
To quantitate the anti-HuD reactivity on the Western blots, we used the Quantity One 1-D Analysis Software Version 4.4.1 (Bio-Rad, Product No. 170–9600). Using this software, we drew a box around the band of interest at 35- to 40-kDa molecular mass in the Western blot image. This same box was copied onto a nearby area with no signal to provide a quantitation for background signal. The background was subtracted from the band-box and this value was used as the adjusted reactivity reading. This was done for each individual Western blot image. Values were set at zero for individuals with an adjusted reading value of zero or less than zero. Western blots were analyzed and quantitated blindly at the same exposure time of five minutes. Representative examples are shown in Figure 1.
Figure 1.
Representative examples of anti-Hu immunoreactivity assays of plasma from SCLC cases and controls. Blots of recombinant human HuD were probed with 1:1000 dilutions of plasma. Lanes 1 and 2: plasma from two SCLC cases. Lanes 3 and 4: plasma from two smoking controls. Lane 5: plasma from a non-smoking control that showed no detectable reactivity. For quantitation, 5 minute exposures were used. For the purposes of this figure only to illustrate that exposure time did not affect reactivity, different exposure times are shown here: 1 min, 40s, 40s, 9 min, and 20 min respectively from left to right.
Follow-Up of Study Population
We performed follow-up of all participants to determine mortality and incidence of new cancers. Using the cut-off date of December, 31, 2003, we checked each study record against information from the SEER Cancer Registry, USC Cancer Surveillance Program of Los Angeles County, for mortality, cause of death, date of death, cancer diagnosis, and date of diagnosis after original study participation.
Statistical Analysis
The SAS statistical software package was used to perform tabular and quantitative analyses [36]. We compared frequency of detection of anti-Hu reactivity according to case-control status. Because the sample sizes were small, we used Fisher’s Exact Test in conjunction with McNemar’s statistic to evaluate departure from expected [37]. We used SAS to fit conditional exact logistic regression models of the association between anti-Hu reactivity and SCLC, adjusting for amount smoked.
To evaluate the association between anti-Hu reactivity and risk of small cell lung cancer, we treated no reactivity as the baseline referent category, or linear zero, and constructed approximate tertiles based on the distribution of antibody positive among the controls (>0 –1800; 1800 – 5400; >5400). The median value of anti-Hu reactivity for each category was used to model tests for trend. Because the logistic regression estimates were not different for the upper two tertiles, we collapsed the anti-Hu reactivity variable into two categories: 0 – 1800 and > 1800 [38].
Case-control differences in levels of covariates were examined with pair-wise ANOVA t-tests for continuous variables, and McNemar’s chi-square statistic for categorical variables [37]. To assess case-control differences in anti-Hu reactivity, we constructed a variable to indicate values of anti-Hu reactivity above zero as ‘detectable’, and values of anti-Hu reactivity equal to zero as ‘undetectable.’ Tests of statistical significance were two sided, and a p-value of less than 0.05 was considered significant.
The potential role smoking might play in relationship to the association between anti-Hu reactivity and SCLC had never before been evaluated. Here our goal was to carefully account for residual confounding from smoking. Due to our small sample size, we chose matching as a method to partially balance our control distribution for smoking history. We matched one set of controls to each case for smoking status (current smoking; former smoking). Because we wanted to compare whether anti-Hu reactivity differed according to smoking, we added a second set of controls who were non-smokers. Models were further adjusted for smoking amount by including indicator terms for total pack years of smoking (< 40 pack years; 40+ pack years).
We conducted survival analysis using the product limit method of estimation for the small cell cases and anti-Hu reactivity in relationship to time to death or censorship since original lung cancer diagnosis. The linear shape of the negative-logarithm plot of survivor function for all small cell cases suggested that the exponential distribution assumptions were met. We constructed survival distribution plots and used likelihood ratio tests to assess difference between survival curves for anti-Hu reactivity. We constructed a Cox proportional hazards regression model adjusting for sex and amount smoked. Additional terms for age and ethnicity added to the proportional hazards model did not improve precision or result in changes to effect estimates, and they were therefore not included in the model.
Results
Characteristics of SCLC cases and controls are summarized in Table 1. The matching factors of race, age, and sex were correspondingly similar between cases and controls. As expected, pack-years of smoking was slightly greater among cases than smoking controls, although this difference was not statistically significant (p = 0.13). Results from the Western blots for anti-Hu reactivity are shown by case-control status in Figure 2.
Table 1.
Distribution of Covariates According to Case-Control Status
| Variable | Category | Cases | Smoking Controls | Non-Smoking Controls | Total Controls | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | p-value1 | ||
| Race | Caucasian | 27 | 65.85 | 25 | 65.79 | 25 | 65.79 | 53 | 67.95 | |
| African American | 14 | 34.15 | 13 | 34.21 | 13 | 34.21 | 25 | 32.05 | 0.82 | |
| Gender | Male | 21 | 51.22 | 19 | 50.00 | 18 | 47.37 | 42 | 53.85 | |
| Female | 20 | 48.78 | 19 | 50.00 | 20 | 52.63 | 36 | 46.15 | 0.79 | |
| Smoking Status | Never | 0 | 0.00 | 0 | 0.00 | 39 | 100.00 | 39 | 50.00 | |
| Former | 8 | 19.51 | 7 | 17.95 | 0 | 0.00 | 7 | 8.97 | ||
| Current | 33 | 80.49 | 32 | 82.05 | 0 | 0.00 | 32 | 41.03 | 0.862 | |
| Age (in years) | < 60 | 15 | 36.59 | 15 | 39.47 | 14 | 36.84 | 28 | 35.90 | |
| 60 – 69 | 20 | 48.78 | 19 | 50.00 | 20 | 52.63 | 41 | 52.56 | ||
| 70 + | 6 | 14.63 | 4 | 10.53 | 4 | 10.53 | 9 | 11.54 | 0.97 | |
| Age (in years) | Mean | 62.46 | 62.31 | 61.68 | 62.31 | |||||
| S.D. | 8.33 | 8.07 | 7.34 | 7.42 | 0.86 | |||||
| Pack years | Mean | 47.17 | 37.66 | 0.00 | 18.83 | |||||
| S.D. | 29.97 | 25.03 | 0.00 | 25.85 | 0.132 | |||||
McNemar exact p-value for categorical data, and pair-wise ANOVA p-values for continuous data from cases and total matched controls (smoking and non-smoking)
McNemar exact p-value for categorical smoking status and paired t-test for mean pack years from cases and matched smoking controls
Figure 2.
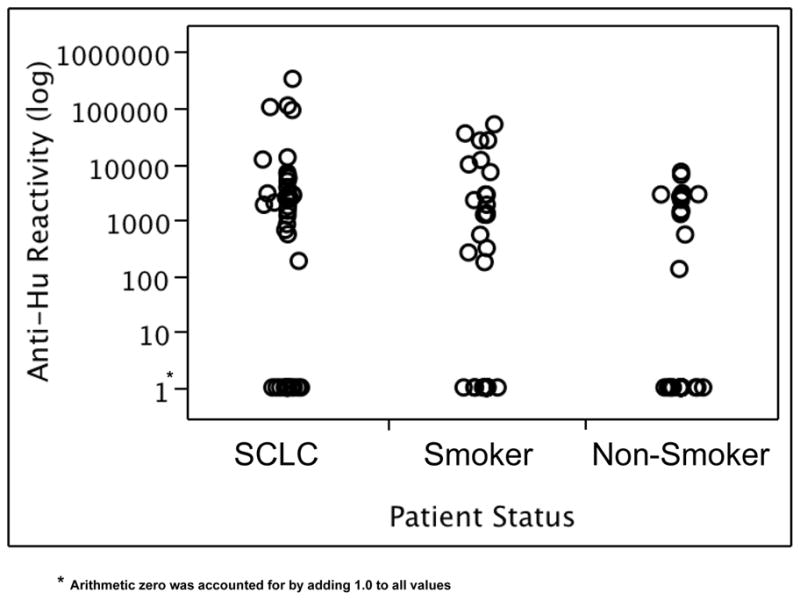
Anti-Hu reactivity levels in SCLC cases, smoking controls and non-smoking controls plotted on a logarithmic scale. To represent arithmetic zero, 1.0 was added to each adjusted reading value.
Table 2 contains the distribution of detectable anti-Hu reactivity for cases and both control groups. Among the total study population, significant differences were observed between cases and control groups (p = 0.01) for detectable anti-Hu reactivity, ranging from 66% among the cases, to 46% among the smoking controls, and 39% among the non-smoking controls. The same trend was observed in the racial, gender, and age subgroups, but was most pronounced in younger subjects (<60 years of age), with cases having 73% detection of anti-Hu; smoking controls with 29% detection; and non-smoking controls with 29% detection (p = 0.02). We also observed significant differences in mean anti-Hu reactivity for cases and controls among Caucasians (p=0.02) and among male subjects (p=0.02).
Table 2.
Frequency of Detectable Anti-Hu Reactivity According to Covariate and Case-Control Status
| Variable | Category | Anti-Hu Reactivity | Cases | Smoking Controls | Non-Smoking Controls | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value1 | |||
| Total Population | Undetectable | 14 | 34.2 | 21 | 53.9 | 24 | 61.5 | ||
| Detectable | 27 | 65.9 | 18 | 46.2 | 15 | 38.5 | 0.01 | ||
| Race | Caucasian | Undetectable | 9 | 33.3 | 14 | 53.9 | 16 | 59.3 | |
| Detectable | 18 | 66.7 | 12 | 46.2 | 11 | 40.7 | 0.02 | ||
| African American | Undetectable | 5 | 35.7 | 7 | 53.9 | 8 | 66.7 | ||
| Detectable | 9 | 64.3 | 6 | 46.2 | 4 | 33.3 | 0.30 | ||
| Gender | Male | Undetectable | 6 | 20.0 | 12 | 60.0 | 12 | 54.6 | |
| Detectable | 15 | 80.0 | 8 | 40.0 | 10 | 45.5 | 0.02 | ||
| Female | Undetectable | 8 | 40.0 | 9 | 47.4 | 12 | 70.6 | ||
| Detectable | 12 | 60.0 | 10 | 52.6 | 5 | 29.4 | 0.21 | ||
| Smoking Status | Former | Undetectable | 2 | 25.0 | 3 | 42.9 | |||
| Detectable | 6 | 75.0 | 4 | 57.1 | 0.662 | ||||
| Current | Undetectable | 12 | 36.4 | 18 | 56.3 | ||||
| Detectable | 21 | 63.6 | 14 | 43.7 | 0.142 | ||||
| Age (in years) | < 60 | Undetectable | 4 | 26.7 | 10 | 71.4 | 10 | 71.4 | |
| Detectable | 11 | 73.3 | 4 | 28.6 | 4 | 28.6 | 0.02 | ||
| 60 – 69 | Undetectable | 8 | 40.0 | 9 | 42.9 | 13 | 65.0 | ||
| Detectable | 12 | 60.0 | 12 | 57.1 | 7 | 35.0 | 0.11 | ||
| 70 + | Undetectable | 2 | 33.3 | 2 | 50.0 | 1 | 20.0 | ||
| Detectable | 4 | 66.7 | 2 | 50.0 | 4 | 80.0 | 0.29 | ||
McNemar exact p-value for categorical data from cases and total matched controls (smoking and non-smoking)
McNemar exact p-value for categorical smoking status from cases and matched smoking controls
In order to evaluate whether smoking was related to anti-Hu reactivity, we compared the frequency of detectable anti-Hu reactivity for the smoking and non-smoking controls (supplementary data1). Anti-Hu reactivity did not vary by smoking for the total sample (p = 0.41) nor did anti-Hu reactivity vary according to racial, or gender subgroups.
Higher anti-Hu reactivity was associated with SCLC, both before and after adjustment for smoking amount (Table 3). The risk of SCLC tended to increase across categories of anti-Hu up to 1800–5400 units, but dropped at the uppermost category, > 5400 units (p for trend = 0.06). We observed a smoking adjusted odds ratio of 3.2 (95 % confidence interval from 0.98 to 13.4) comparing subjects with anti-Hu reactivity > 1800 to subjects with lower reactivity.
Table 3.
Conditional 1 exact odds ratios describing the association between anti-Hu reactivity and small cell lung carcinoma
| Variable | Category | Cases | Controls | OR 2 | OR 3 | 95% Confidence Interval 3 | p-value4 |
|---|---|---|---|---|---|---|---|
| Anti-Hu Reactivity | 0 | 14 | 45 | 1.00 | 1.00 | ----------- | |
| > 0 – 1800 | 7 | 13 | 1.96 | 0.74 | 0.10 – 4.40 | ||
| 1800 – 5400 | 11 | 11 | 3.55 | 5.44 | 1.06 – 54.36 | ||
| > 5400 | 9 | 9 | 3.94 | 1.77 | 0.39 – 9.39 | 0.06 | |
| Anti-Hu Reactivity | 0 – 1800 | 21 | 58 | 1.00 | 1.00 | ------------ | |
| > 1800 | 20 | 20 | 3.10 | 3.17 | 0.98 – 13.38 |
Cases matched to controls for age (five-year intervals), race, sex, and smoking status
Unadjusted
Adjusted using indicator variables for pack-years
Score Test Probability
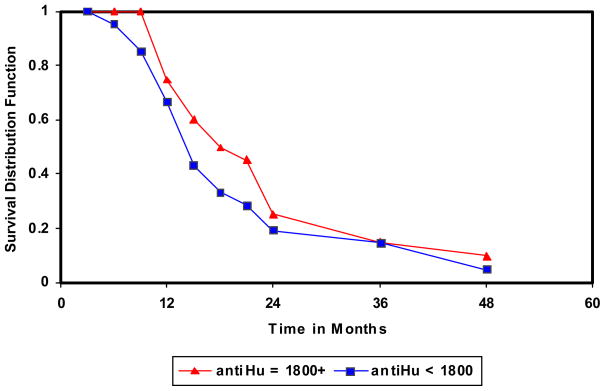
We conducted follow-up on small cell lung cancer cases, in order to determine whether anti-Hu reactivity was related to prognosis. Figure 3 contains product-limit estimates of survival from small cell lung cancer according to anti-Hu reactivity. Cases with an anti-Hu reactivity level > 1800 units survived longer (median survival time = 15.6 months) than cases below 1800 anti-Hu reactivity level (median survival time = 11.4 months) (p = 0.08). The follow-up period was too short with too few controls to assess whether anti-Hu reactivity might be associated with future development of small cell and other cancers.
Figure 3.
Plot of time until death or censorship. Plots of survival functions for anti-Hu reactivity; above and below 1800 units.
X-axis indicates time in months. Y axis indicates survival distribution function.
We further examined the association between survival time and anti-Hu reactivity by constructing a Cox proportional-hazards regression model and estimating hazard-rate ratios for the probability of surviving. The hazard rate ratio in relation to anti-Hu reactivity levels above 1800 units was 0.46 (95% confidence interval = 0.23 to 0.93) compared to below 1800 units, adjusting for sex and smoking amount.
Discussion
This is the first examination of anti-Hu reactivity in relation to lung cancer risk in a population-based study. To sensitively determine levels of anti-Hu reactivity in SCLC cases and control subjects, we used recombinant HuD protein in a Western blot analysis, using Biorad Quantity One 1-D Analysis Software to quantitate 5-min exposures in a blinded fashing. Only reactivity against the correct molecular weight band was scored as positive. This assay allows the sensitive and specific unbiased detection of even low titers of anti-Hu antibodies while minimizing false positives that may be obtained with other methods [35]. For example, as was done in the Dalmau study, the use of crude neuronal extracts may lead to scoring of sera with reactivity against proteins other than Hu [21;39], while in ELISA the size of the reacting protein is not visible. The use of imaging software likely increased our sensitivity compared to other studies, which often score samples using a more subjective scals (e.g. “mild, moderate, intense”) (see below).
In our study, we did not establish a ‘reactivity’ cut point based on reactivity levels in control subjects. Instead our goal was to measure the direct level of anti-Hu reactivity in all subjects and evaluate associations between level of anti-Hu reactivity and SCLC. In contrast. Dalmau and colleagues considered reactivity of normal human sera (equivalent to 6U/ml) as background; sera from SCLC patients with and without PEM/SN were considered positive only if their reactivity was greater than twice the background value found in normal sera. Seven of 44 (16%) SCLC patients without PEM/SN had detectable levels of anti-Hu antibody (average 76 U/ml) [21]. Other studies that used recombinant HuD antigen on Western blots created categories of mild, moderate, or intense to describe anti-Hu reactivity [10;22]. These studies identified positive reactivity in 16% of their total SCLC population [10], and 17% of SCLC cases [22]. Guidelines for detection of anti-Hu antibodies established by the International Society of Neuro-Immunology noted that specimens yielding no reactivity on immunohistochemistry may nonetheless be detected by Western blot analysis using highly affinity purified recombinant proteins, suggesting that these methods may be more sensitive [35]. When using a highly sensitive in vitro transcription-translation (ITT) assay and immunoprecipitation, Monstad et al, observed anti-Hu reactivity in 25.5% of SCLC patients. When they submitted the same specimens to a dot blot analysis using recombinant HuD and 1:2000 dilution, they found results (18.5%) comparable to previous studies [23].
Methods for classifying reactivity and detection methods thus appear to impact prevalence of anti-Hu reactivity. To make compare our analysis to other studies, we could consider the highest anti-Hu reactivity value in the non-smokers as our baseline cutoff value (~7500). In cutting our reactivity at 7500 and considering values above 7500 as positive, we found that 15% of SCLC patients had detectable antibody levels. This finding is similar to previous results [10;21;22], but, in our opinion, negates the important finding that anti-Hu reactivity is detectable at values lower than 7500 among smoking and non-smoking controls.
We found a moderate association between anti-Hu reactivity and SCLC. The odds ratio for moderate to high positive anti-Hu reactivity (>1800 units), adjusted for total pack years of smoking, was marginally statistically significant (odds ratio = 3.2 (95% confidence interval = 0.98 to 13.4)).
Examining the frequency of detectable anti-Hu reactivity, we observed an unadjusted association between anti-Hu reactivity and case-control status (p = 0.01). We adjusted for total pack years of smoking using analysis of covariance (ANCOVA), and the association between continuous anti-Hu reactivity and case-control status was significant (p = 0.05). We compared anti-Hu reactivity among the controls according to smoking status. We found no overall difference and no differences when we stratified by gender and race. In the retrospective studies by Dalmau and Verschuuren, there was a predominance of women who were anti-Hu positive [21;22]. Graus et al observed no difference in mean age and sex in SCLC patients with and without PEM/SN [10]. When comparing patients positive or negative for anti-Hu antibodies, Monstad et al reported no significant differences between sex or age [23]. These studies did not however examine anti-Hu reactivity according to race.
We observed that anti-Hu reactivity was protective for survival from small cell lung cancer in our study, however results can only be considered suggestive due to small numbers. Our results are in agreement with the previously observed longer survival time of anti-Hu positive SCLC patients [22], the more limited disease observed in anti-Hu positive SCLC cases [21], and reports about small cell lung cancer cases with paraneoplastic sensory neuronopathy in complete remission [40;41]. The presence of anti-Hu antibodies may indicate an immune system response to small cell lung cancer that could function to prolong survival.
Our study differs from previously conducted research on anti-Hu antibodies and SCLC in that we used healthy population-based controls matched on age, race, sex, and smoking status. Large scale studies are needed to determine whether anti-Hu reactivity profiles can serve as an early detection marker for SCLC. Interestingly,, one of the smoking controls with a detectable level of anti-Hu antibody, healthy at time of study participation, died of SCLC several years after the study was concluded. This observation supports a possibility that moderate level anti-Hu reactivity might be an indicator for future SCLC. While this is an anecdotal occurrence, appearance of a small cell lung cancer case in a control group of this size would not be expected given the underlying background of SCLC incidence in Los Angeles County that occurred during the study period [3;42].
We found that smoking was not associated with anti-Hu reactivity among our healthy controls. Smoking greatly increases the risk of small cell lung cancer. Development of anti-Hu reactivity, on the other hand, may signal the presence of a small cell lung tumor, but it is not necessarily related to smoking.
Small cell lung cancer is one of the most rapidly fatal cancers [3;9]. Identification of high-risk smokers that may develop SCLC could help to improve survival. Smokers who are determined to be at risk for SCLC could be closely monitored and could serve as candidates for chemoprevention once effective agents become available. In addition, they should strongly be encouraged to quit smoking. Although only suggestive, given our small sample size, reactivity against Hu proteins and other auto-antigens could indicate the first signs of a growing small cell lung tumor, potentially in time to prolong survival.
Supplementary Material
Acknowledgments
Sources of Support: Establishment of the case-control study and specimen collection was funded by the California Tobacco Related Disease Research Program (TRDRP) grants, 1RT-140, and 3RT-403. Follow-up and data analysis was supported by TRDRP grant, 11IT-0082. Generous donations from Eri and Mary Lou Mettler supported the laboratory analysis. Dr. London is supported by the Division of Intramural Research, National Institute of Environmental Health Sciences, NIH, DHHS.
The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Supplementary Table 1 will be published only in the journal’s “on-line” version.
References
- 1.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001 Jul 18;93(14):1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 2.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001 Apr;28(2 Suppl 4):3–13. [PubMed] [Google Scholar]
- 3.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. Lung-Bronchus, Percent Distribution and Counts by Histology, 2001–2004, Both Sexes by Race, Table XV-23. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2004. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission posted to the SEER web site 2007 [cited 2007 Sep 8]. Available from: http://seer.cancer.gov/csr/1975_2004/results_merged/sect_15_lung_bronchus.pdf. [Google Scholar]
- 4.Lubin JH, Blot WJ. Assessment of lung cancer risk factors by histologic category. J Natl Cancer Inst. 1984 Aug;73(2):383–9. doi: 10.1093/jnci/73.2.383. [DOI] [PubMed] [Google Scholar]
- 5.Morabia A, Wynder EL. Cigarette smoking and lung cancer cell types. Cancer. 1991 Nov 1;68(9):2074–8. doi: 10.1002/1097-0142(19911101)68:9<2074::aid-cncr2820680939>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberg JB, Wilcox HB, Mason TJ, Bill J, Stemhagen A. Variation in smoking-related lung cancer risk among New Jersey women. Am J Epidemiol. 1989 Oct;130(4):688–95. doi: 10.1093/oxfordjournals.aje.a115390. [DOI] [PubMed] [Google Scholar]
- 7.Wynder EL, Kabat GC. The effect of low-yield cigarette smoking on lung cancer risk. Cancer. 1988 Sep 15;62(6):1223–30. doi: 10.1002/1097-0142(19880915)62:6<1223::aid-cncr2820620630>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZY, Blot WJ, Xiao HP, Wu A, Feng YP, Stone BJ, et al. Smoking, air pollution, and the high rates of lung cancer in Shenyang, China. J Natl Cancer Inst. 1989 Dec 6;81(23):1800–6. doi: 10.1093/jnci/81.23.1800. [DOI] [PubMed] [Google Scholar]
- 9.Sandler AB. Chemotherapy for small cell lung cancer. Semin Oncol. 2003 Feb;30(1):9–25. doi: 10.1053/sonc.2003.50012. [DOI] [PubMed] [Google Scholar]
- 10.Graus F, Dalmou J, Rene R, Tora M, Malats N, Verschuuren JJ, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997 Aug;15(8):2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 11.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992 Oct;141(4):881–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, et al. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991 Oct 18;67(2):325–33. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 13.Good PJ. A conserved family of elav-like genes in vertebrates. Proc Natl Acad Sci U S A. 1995;92:4557–61. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara S, et al. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9885–90. doi: 10.1073/pnas.96.17.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball NS, King PH. Neuron-specific hel-N1 and HuD as novel molecular markers of neuroblastoma: a correlation of HuD messenger RNA levels with favorable prognostic features. Clin Cancer Res. 1997 Oct;3(10):1859–65. [PubMed] [Google Scholar]
- 16.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997 May 1;17(9):3024–37. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graus F, Cordon-Cardo C, Posner JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985 Apr;35(4):538–43. doi: 10.1212/wnl.35.4.538. [DOI] [PubMed] [Google Scholar]
- 18.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu--associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992 Mar;71(2):59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Dalmau J, Graus F, Cheung NK, Rosenblum MK, Ho A, Canete A, et al. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995 Jan 1;75(1):99–109. doi: 10.1002/1097-0142(19950101)75:1<99::aid-cncr2820750117>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Manley GT, Smitt PS, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995 Jul;38(1):102–10. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer--a quantitative western blot analysis. Ann Neurol. 1990 May;27(5):544–52. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 22.Verschuuren JJ, Perquin M, ten Velde G, DeBaets M, van Breda Vriesman P, Twijnstra A. Anti-Hu antibody titre and brain metastases before and after treatment for small cell lung cancer. J Nneurol Neurosurg Psychiatry. 1999;67:353–357. doi: 10.1136/jnnp.67.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monstad SE, Drivsholm L, Storstein A, Aurseth JH, Haugen M, Lang B, Vincent A, Vedeler CA. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small cell lung cancer. J Clin Oncol. 2004;22:795–800. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Meriney SD, Hulsizer SC, Lennon VA, Grinnell AD. Lambert-Eaton myasthenic syndrome immunoglobulins react with multiple types of calcium channels in small-cell lung carcinoma. Ann Neurol. 1996 Nov;40(5):739–49. doi: 10.1002/ana.410400510. [DOI] [PubMed] [Google Scholar]
- 25.Oguro-Okano M, Griesmann GE, Wieben ED, Slaymaker SJ, Snutch TP, Lennon VA. Molecular diversity of neuronal-type calcium channels identified in small cell lung carcinoma. Mayo Clin Proc. 1992 Dec;67(12):1150–9. doi: 10.1016/s0025-6196(12)61144-6. [DOI] [PubMed] [Google Scholar]
- 26.Alamowitch S, Graus F, Uchuya M, Rene R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997 Jun;120( Pt 6):923–8. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 27.Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain. 1968 Sep;91(3):481–96. doi: 10.1093/brain/91.3.481. [DOI] [PubMed] [Google Scholar]
- 28.Bataller L, Graus F, Saiz A, Vilchez JJ. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001 Feb;124(Pt 2):437–43. doi: 10.1093/brain/124.2.437. [DOI] [PubMed] [Google Scholar]
- 29.Bazhin AV, Shifrina ON, Savchenko MS, Tikhomirova NK, Goncharskaia MA, Gorbunova VA, et al. Low titre autoantibodies against recoverin in sera of patients with small cell lung cancer but without a loss of vision. Lung Cancer. 2001 Oct;34(1):99–104. doi: 10.1016/s0169-5002(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 30.Thirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JL. Intraperitoneal cultivation of small-cell carcinoma induces expression of the retinal cancer-associated retinopathy antigen. Arch Ophthalmol. 1993 Jul;111(7):974–8. doi: 10.1001/archopht.1993.01090070094026. [DOI] [PubMed] [Google Scholar]
- 31.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003 Oct 16;349(16):1543–54. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 32.London SJ, Daly AK, Cooper J, Navidi WC, Carpenter CL, Idle JR. Polymorphism of glutathione S-transferase M1 and lung cancer risk among African-Americans and Caucasians in Los Angeles County, California. J Natl Cancer Inst. 1995 Aug 16;87(16):1246–53. doi: 10.1093/jnci/87.16.1246. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Myszka DG, Yu M, Littler SJ, Laird-Offringa IA. HuD RNA recognition motifs play distinct roles in the formation of a stable complex with AU-rich RNA. Mol Cell Biol. 2000 Jul;20(13):4765–72. doi: 10.1128/mcb.20.13.4765-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moll JW, Antoine JC, Brashear HR, Delattre J, Drlicek M, Dropcho EJ, et al. Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies: report from the Workshop to the Fourth Meeting of the International Society of Neuro-Immunology on paraneoplastic neurological disease, held October 22–23, 1994, in Rotterdam, The Netherlands. Neurology. 1995 Oct;45(10):1937–41. doi: 10.1212/wnl.45.10.1937. [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute. SAS Statistical Software, version 9.1.3. SAS Institute 2005
- 37.Fleiss J. Statistical methods for rates and proportions. 2. New York: John Wiley & Sons; 1980. [Google Scholar]
- 38.Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 39.Sillevis-Smitt P, Manley G, Moll JW, Dalmau J, Posner JB. Pitfalls in the diagnosis of autoantibodies associated with paraneoplastic neurologic disease. Neurology. 1996 Jun;46(6):1739–41. doi: 10.1212/wnl.46.6.1739. [DOI] [PubMed] [Google Scholar]
- 40.Gill S, Murray N, Dalmau J, Thiessen B. Paraneoplastic sensory neuronopathy and spontaneous regression of small cell lung cancer. Can J Neurol Sci. 2003 Aug;30(3):269–71. doi: 10.1017/s0317167100002729. [DOI] [PubMed] [Google Scholar]
- 41.Horino T, Takao T, Yamamoto M, Geshi T, Hashimoto K. Spontaneous remission of small cell lung cancer: a case report and review in the literature. Lung Cancer. 2006 Aug;53(2):249–52. doi: 10.1016/j.lungcan.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute, Surveillance, Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 13 Regs Public-Use, Nov 2006 Sub (1992–2004) Bethesda MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; released April 2007 based on November 2006 submission [cited 2007 Sep 8]. Available from: http://seer.cancer.gov/canques/incidence.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.