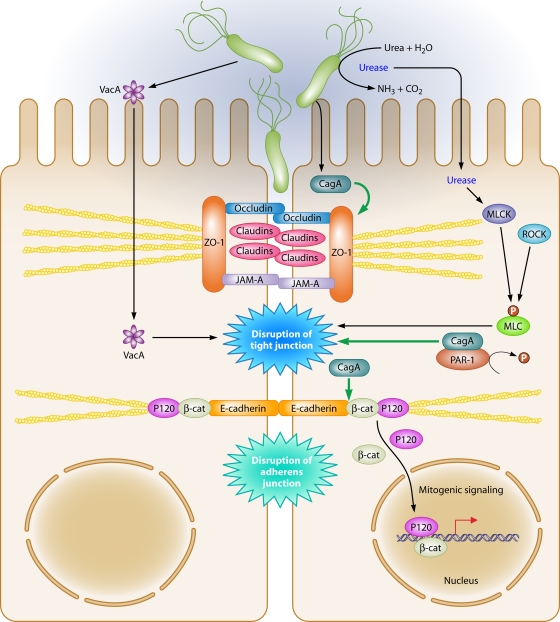
FIG. 5.
Dysregulation of the apical-junctional complex by H. pylori. H. pylori preferentially adheres to the apical-junctional complex of epithelial cells and alters localization of apical-junctional component proteins, disrupts epithelial barrier function, cell adhesion, and cell polarity, and induces an invasive phenotype. Translocated CagA interacts with PAR1, preventing phosphorylation of PAR1 by blocking PAR1 kinase activity, which culminates in disruption of the tight junction. In addition, functional urease activity can disrupt the tight junction via a mechanism involving MLC phosphorylation, which can be regulated by MLCK and Rho kinase. VacA can also increase tight junction permeability to low-molecular-weight molecules and ions.