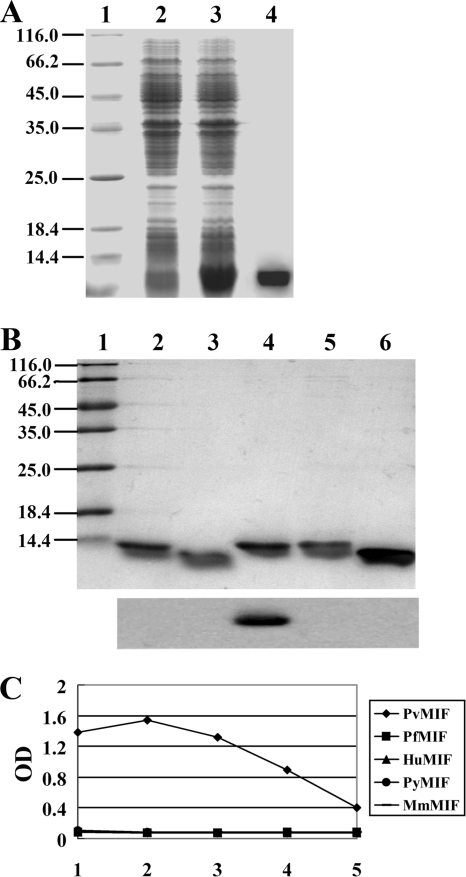
FIG. 1.
Purification of recombinant PvMIF and specificity identification of monoclonal antibody 3B4. (A) Protein extracted from preinduced (lane 2) and induced (lane 3) bacteria carrying pET 30a-Pvmif are shown. The induced extract was passed over a nickel affinity column, and the 13.4-kDa rPvMIF-His fusion protein was eluted with imidazole (lane 4). (B) Purified recombinant PyMIF (rPyMIF) (lane 2), rMmMIF (lane 3), rPvMIF (lane 4), rPfMIF (lane 5), and rHuMIF (lane 6) proteins are shown. All of the proteins were transferred to a polyvinyl difluoride membrane and immunostained with MAb 3B4 (bottom). All of the extracts and proteins were separated by 15% SDS-PAGE, and a low-molecule-weight protein marker is shown (lane 1). (C) Specificity detection of MAb 3B4 by ELISA. Purified rPvMIF, rPfMIF, rHuMIF, rPyMIF, and rMmMIF were coated. The dilutions of MAb 3B4 were the following: lane 1, 1:500; 2, 1:2,500; 3, 1:12,500; 4, 1:62,500; 5, 1:312,500.