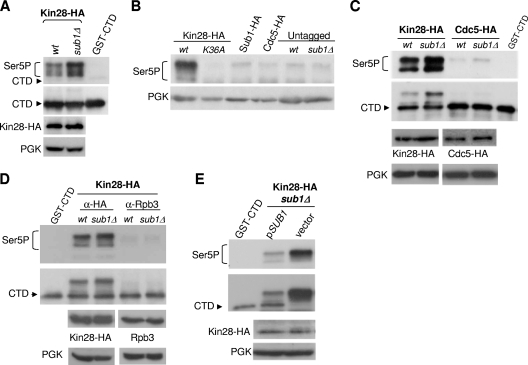
FIG. 1.
SUB1 deletion increases Kin28 CTD kinase activity and Kin28 cross-linking to gene promoters. (A) In vitro kinase assay. Whole-cell extracts were prepared from wild-type (wt) and sub1Δ strains expressing HA-tagged Kin28. The epitope-tagged kinase complexes were immunoprecipitated with 12CA5-protein A beads, and kinase activity was assayed with 2.5 mM ATP and recombinant GST-CTD as substrate. SDS-PAGE and immunoblot analysis were performed to analyze CTD phosphorylation, using the following antibodies: CTD4H8 (anti-CTD Ser5P), 8WG16 (anti-CTD), 12CA5 (anti-HA, for Kin28-HA), and PGK (anti-PGK, as total protein level control). (B) Whole-cell extracts were prepared from the following strains: tagged Kin28-HA (wt and kin28-K36A mutant), Sub1-HA, and Cdc5-HA strains and two nontagged strains (wt and sub1Δ). In vitro kinase assays were conducted as described for panel A, and CTD Ser5 phosphorylation analyzed with CTD4H8 antibody. (C) Whole-cell extracts were prepared from wt and sub1Δ strains expressing HA-tagged Cdc5, and in vitro kinase assays performed to analyze CTD Ser5P as described above. (D) In vitro kinase assay to analyze CTD Ser5 phosphorylation was performed using immunoprecipitated Kin28-HA and Rpb3 from wt and sub1Δ Kin28-HA tagged strains. (E) Kin28-HA sub1Δ cells were transformed with an empty plasmid (vector) or with a plasmid bearing SUB1 (pSUB1) under the control of its own promoter. Kin28-HA was immunoprecipitated, kinase assays performed, and CTD Ser5P analyzed as described for panel B.