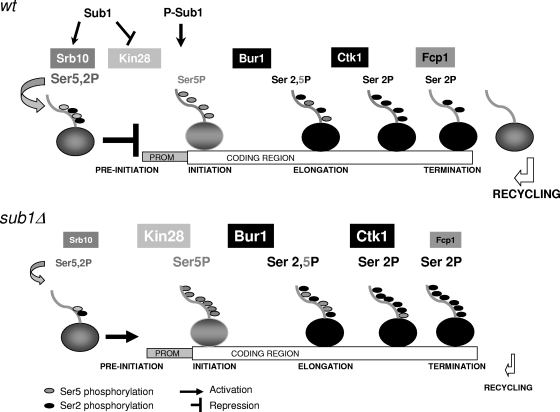
FIG. 6.
Model showing how Sub1 might function to regulate RNAP II CTD phosphorylation. In wild-type cells, nonphosphorylated Sub1 joins the promoter (PROM) (possibly via TFIIB [see references 28, 37, and 60]), contacting the promoter via its DNA binding domain. At that point, Sub1 interacts with the CDK8 (Srb10) Mediator complex, helping to maintain the PIC in a stable but inactive conformation. Sub1 is then phosphorylated (possibly by the action of kinases at the PIC, similarly to PC4), losing its DNA binding capacity and promoting clearance of TFIIB (26, 35). The PIC next changes conformation such that Kin28 can be activated and, with the help of Srb10, promotes PIC dissociation into the scaffold complex, as well as the recruitment of elongating kinases Ctk1 and Bur1. In contrast, in sub1Δ cells, Srb10 activity and recruitment are decreased, while Kin28 recruitment and activity increase, in agreement with TFIIH being negatively regulated by CDK8-containing Mediator complexes (4, 51). As a result, Ser5P levels are increased, and consequently, Bur1 and Ctk1 association with chromatin is also enhanced (19, 57). Furthermore, in sub1Δ cells, there is a reduction in Fcp1 phosphatase levels and its association with chromatin, which induces an additional increase in Ser2P, impairing RNAP II recycling after transcription termination. Thus, a decrease in RNAP II recruitment is observed in cells lacking Sub1 (11). Different font sizes in the figure labels indicate the increase or decrease of the corresponding CTD-modifying enzymes in sub1Δ versus wt cells.