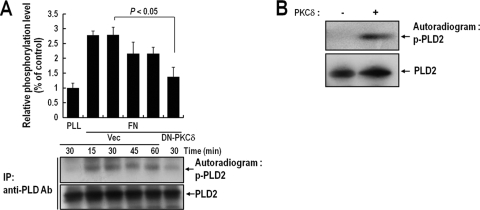
FIG. 3.
PKCδ phosphorylates PLD2 during integrin-mediated cell spreading. (A) COS-7 cells were cotransfected with PLD2 and either a vector control (Vec) or DN-PKCδ. One day after transfection, cells were serum starved for 20 h; then they were loaded with 32Pi (1 mCi/ml) for 4 h. Cells were then detached from culture dishes and were maintained in suspension for 3 h. They were then replated on FN or PLL for the indicated times (15, 30, 45, or 60 min). “PLL” represents the control and indicates the phosphorylation level of PLD2 in adherent cells on PLL at 30 min. Cells were harvested, and PLD was immunoprecipitated (IP) with an anti-pan-PLD antibody (Ab). Immunoprecipitated proteins were separated by 8% SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The amounts of PLD2 bound to the immune complex were analyzed by immunoblotting with an anti-pan-PLD antibody. The results shown are representative of three independent experiments. (B) A 200 pM concentration of purified PLD2 protein was immobilized with an anti-pan-PLD antibody coupled to protein A resin, and this complex was incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. Reaction mixtures were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The amounts of PLD2 bound to the immune complex were analyzed by immunoblotting with an anti-pan-PLD antibody. The results shown are representative of three separate experiments.