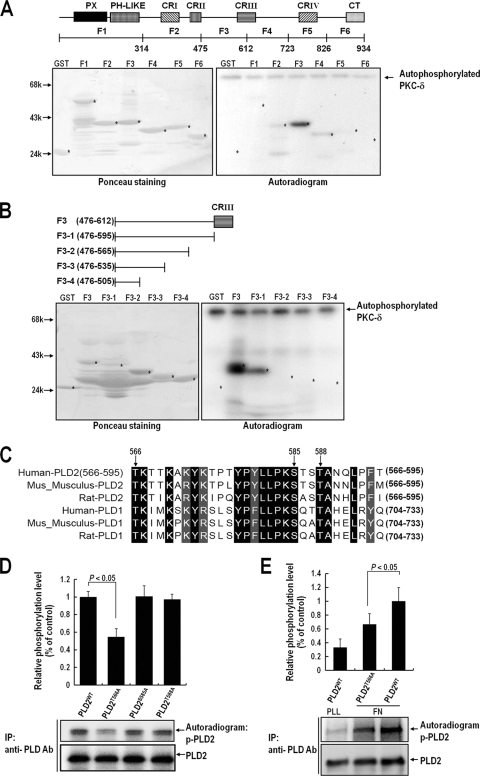
FIG. 4.
Thr566 of PLD2 is phosphorylated by PKCδ in vivo and in vitro. (A) Equal amounts (1 μg) of purified GST or GST fragments of PLD2 were immobilized on glutathione-Sepharose and incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. (Right) Reaction mixtures were then subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. (Left) The amounts of GST fusion proteins on nitrocellulose membranes were quantified by Ponceau staining. The data shown are representative of two independent experiments. (B) Equal amounts (1 μg) of purified GST or GST fragments of the F3 region of PLD2 (amino acids 476 to 612) were immobilized on glutathione-Sepharose and incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. (Right) Reaction mixtures were then subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. (Left) The amounts of GST fusion proteins on nitrocellulose membranes were quantified by Ponceau staining. The data shown are representative of two independent experiments. (C) Sequences of human, rat, and mouse PLD1 and PLD2 (all homologous with respect to amino acids 566 to 595 of human PLD2) were aligned using Clustal W software. Identical amino acids are indicated by dark shaded boxes, and homologous amino acids are indicated by light shaded boxes. PLD2 and the other isoforms were found to be identical with respect to the serine/threonine sequence consisting of Thr566, Ser585, and Thr588 between amino acids 566 and 595, as indicated by the arrows. (D) COS-7 cells were transfected with either PLD2WT, PLD2T566A, PLD2S585A, or PLD2T588A. One day after transfection, cells were serum starved for 24 h and were then harvested. PLD was immunoprecipitated (IP) with an anti-pan-PLD antibody (Ab). The immune complex obtained was incubated with purified PKCδ (100 pM) in phosphorylation buffer, and the reaction mixture was then subjected to SDS-PAGE. Blots were transferred to nitrocellulose membranes and were exposed to photographic film. The amounts of PLD2WT and PLD2 mutants bound to the immune complex were quantified by immunoblotting with an anti-pan-PLD antibody. The levels of phosphorylated PLD determined by densitometry were normalized to the levels of precipitated PLD protein. Data are means; error bars, SD (n = 3). (E) COS-7 cells were transfected with PLDWT or PLD2T566A. After 24 h of transfection, cells were serum starved for 20 h, after which they were loaded with 32Pi (1 mCi/ml) for 4 h. After cells were detached from culture dishes, they were maintained in suspension for 3 h. Cells were then replated on FN or PLL for 20 min and were harvested. PLD was immunoprecipitated using an anti-pan-PLD antibody. Immunoprecipitated proteins were subjected to 8% SDS-PAGE, transferred to nitrocellulose membranes, exposed to a phosphorimager plate, and read with an autoradiography imaging system (FLA-5000; Fujifilm). The amounts of PLD2 bound to the immune complex were determined by immunoblotting with an anti-pan-PLD antibody. The levels of phosphorylated PLD determined by densitometry were normalized to the levels of precipitated PLD protein. Data are means; error bars, SD (n = 3).