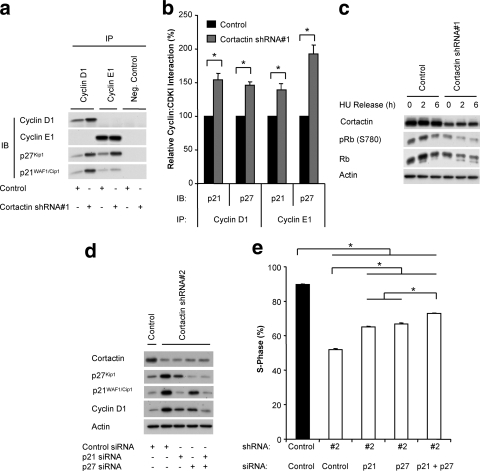
FIG. 4.
Determination of the functional role of p21WAF1/Cip1 and p27Kip1 downstream of cortactin. (A) Coimmunoprecipitation analysis. Cyclin D1 and cyclin E1 were immunoprecipitated from lysates of control and cortactin knockdown cells that had been synchronized and released for 4 h. Western blotting was undertaken to detect the coimmunoprecipitation of p21WAF1/Cip1 and p27Kip1. (B) Quantification of coimmunoprecipitation data. The levels of associating p21WAF1/Cip1 or p27Kip1 were normalized for the amount of cyclin immunoprecipitated and then expressed relative to the value for control cells, which was arbitrarily set at 100%. Data represent the mean ± standard error of three independent experiments. *, P < 0.05. (C) Rb phosphorylation. Control and cortactin knockdown cells were synchronized into G1 phase and released for the time points indicated. Cell lysates were subjected to Western blotting with the indicated antibodies. Data shown are representative of at least three independent experiments. HU, hydroxyurea. (D) Reduction in p21WAF1/Cip1 and p27Kip1 expression levels using specific siRNAs. These CDKIs were transiently depleted by the addition of specific siRNAs during the serum starvation step of the synchronization procedure. Following a 6-h release, Western blotting was undertaken as indicated. Data shown are representative of at least three independent experiments. (E) Reduction of p21WAF1/Cip1 and p27Kip1 expression enhances cell cycle progression in cortactin knockdown cells. Entry into S-phase after a 6-h release was determined by uptake of BrdU during a 2 h pulse. The incorporation of BrdU was measured by flow cytometry. Data represent the mean ± standard error of three independent experiments. *, P < 0.05).