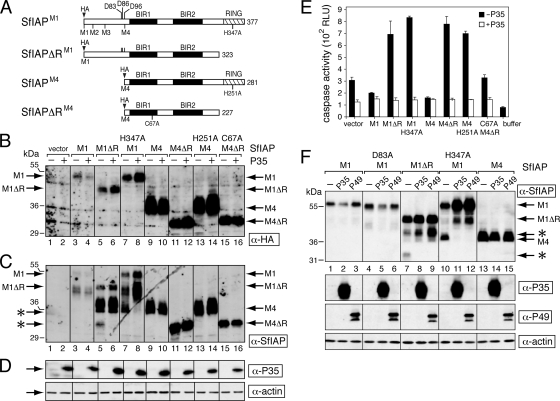
FIG. 9.
Stability of SfIAPM1 and dominant inhibition. (A) SfIAPs. SfIAPM1 (377 residues) and SfIAPM4 (281 residues) begin from the first and fourth methionine, respectively, of the predicted Sfiap ORF. SfIAPΔRM1 and SfIAPΔRM4 each lack their C-terminal RING domain (cross-hatched box). N-terminal epitope tags (HA) were included. Only SfIAPM1 and SfIAPΔRM1 possess the caspase cleavage site at Asp83. (B to D) Protein levels. SF21 cells were transfected with empty vector or plasmids encoding the indicated SfIAPM1 (M1) or SfIAPM4 (M4) proteins with (+) or without (−) an expression vector encoding the caspase inhibitor P35. After 24 h, the cells were lysed and subjected to immunoblot analysis by using anti-HA (B), anti-SfIAP (C), or anti-P35 and anti-actin (D). Each panel represents a single, contiguous immunoblot. SfIAPM1- and SfIAPM4-related proteins are indicated. Caspase cleavage products (*) are indicated on the left. (E) Intracellular caspase activity. Extracts prepared from cells transfected with or without p35 as described in the legend to panel B were assayed for caspase activity by using substrate DEVD-AMC. Values are reported as the averages ± standard deviations in relative light units (RLU) determined from triplicate transfections. Buffer, lysis buffer alone. (F) Effect of caspase inhibitor P49. SF21 cells were transfected with plasmid encoding the indicated SfIAPM1 (M1) or SfIAPM4 (M4) proteins along with empty vector (−) or plasmids encoding P35 or P49. The effector caspase cleavage site at Asp83 was eliminated in D83A-mutated SfIAPM1. Immunoblot analysis of 24-h lysates was performed using the indicated antisera; each panel is a contiguous immunoblot. Caspase cleavage products (*) are indicated on the right.