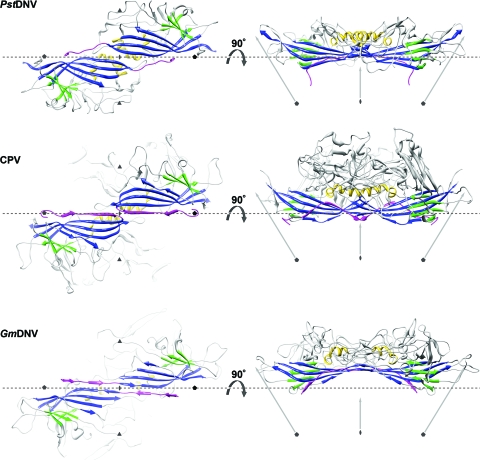
FIG. 2.
Spatial arrangement of the parvoviral core jelly roll and the N-terminal region for the capsid proteins of PstDNV, CPV, and GmDNV. (Left) Two twofold-related symmetry mates are shown, viewed from the viral center along an icosahedral twofold axis. (Right) The side view of the dimer, turned by 90° around the broken-line axis, demonstrates the spatial outward displacement of the β-barrel in GmDNV. Conserved secondary structure elements of each protein subunit are colored blue (β-BIDG), green (β-CHEF), and gold (helical elements). The N-terminal region of the capsid protein, upstream of βB, including βA, is shown in magenta. The positions of icosahedral symmetry axes are indicated by polygonal symbols and arrows. The spatial position of the broken line connecting two neighboring fivefold axes is the same for the three viruses to allow positional comparison of the structures.