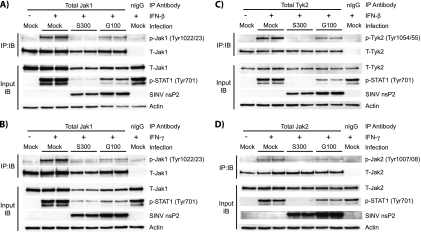
FIG. 3.
Reduced STAT1 phosphorylation in S300-infected cells correlates with defects in Jak activation by type I and type II IFNs. Vero cells were infected (MOI = 10 PFU/cell) with S300, G100, or diluent alone (mock) and then treated for 20 min with 1,000 U IFN-β (A and C) or IFN-γ (B and D) per ml. Cell extracts were then prepared as described in Materials and Methods, and total protein content was quantified. Equal amounts of total protein were then subjected to immunoprecipitation with antibodies directed against total Jak1 (A and B), total Tyk2 (C), or total Jak2 (D), and to assess activation, the immunoprecipitates were resolved by SDS-PAGE and immunoblotted using the indicated phospho-specific Jak antibody (IB:IP panels). These blots were then stripped and reprobed using an antibody recognizing the appropriate total Jak. A portion of the input lysates (5 to 7%) was subjected to direct immunoblot analysis to assess total Jak protein levels (input IB panels). These blots were stripped and reprobed consecutive times with phospho-specific STAT1 (Tyr701) and anti-SINV nsP2 polyclonal serum. The data shown in panel A are representative of 5 independent experiments. Data in panels B to D are representative of at least 2 experiments totaling at least 4 independent samples per infection group.