Abstract
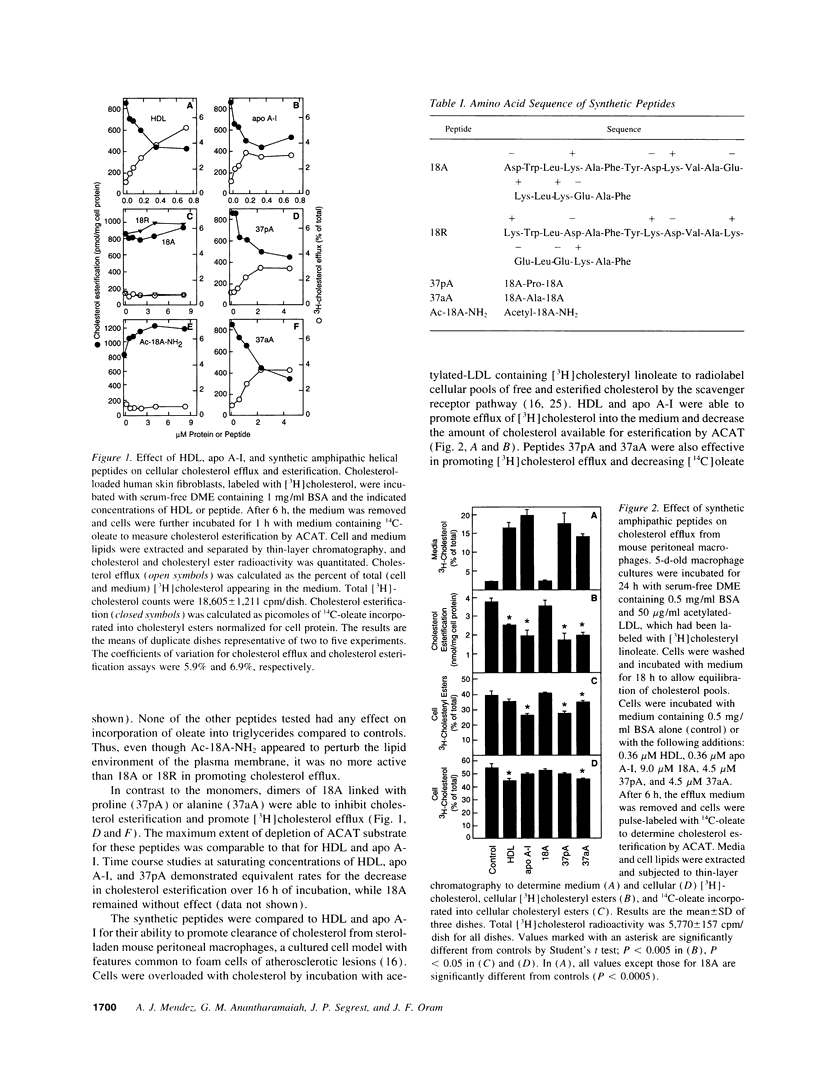
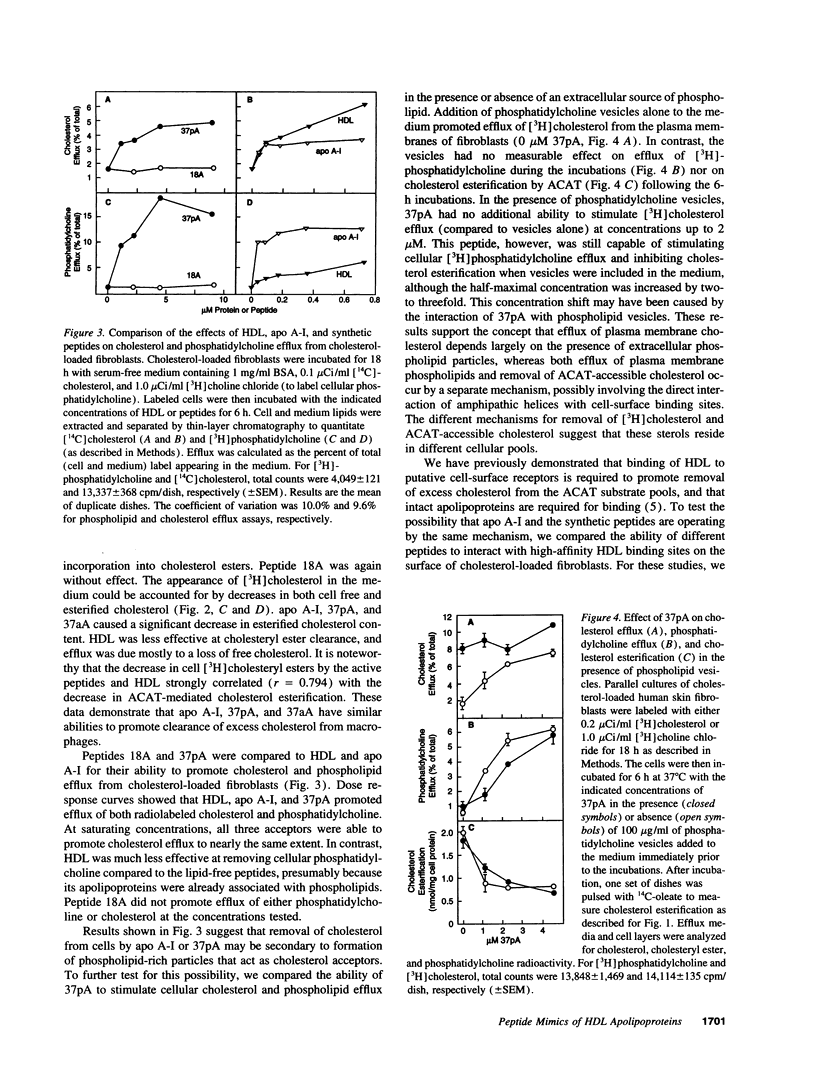
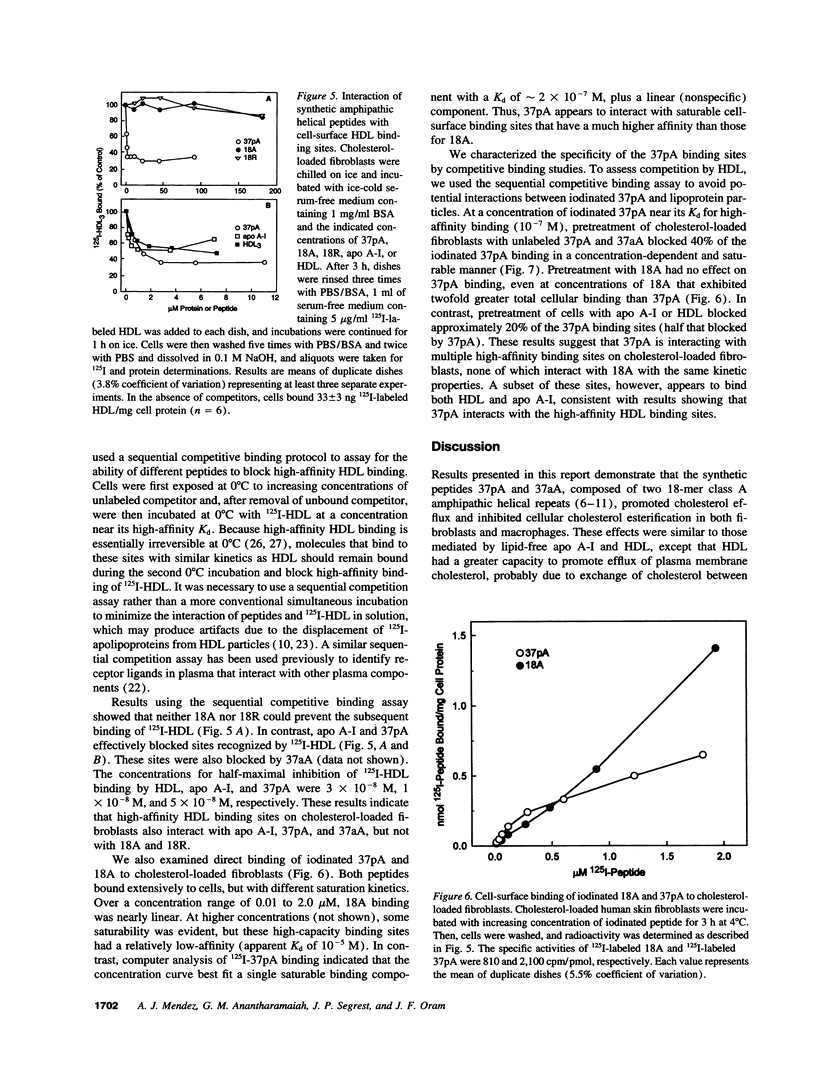
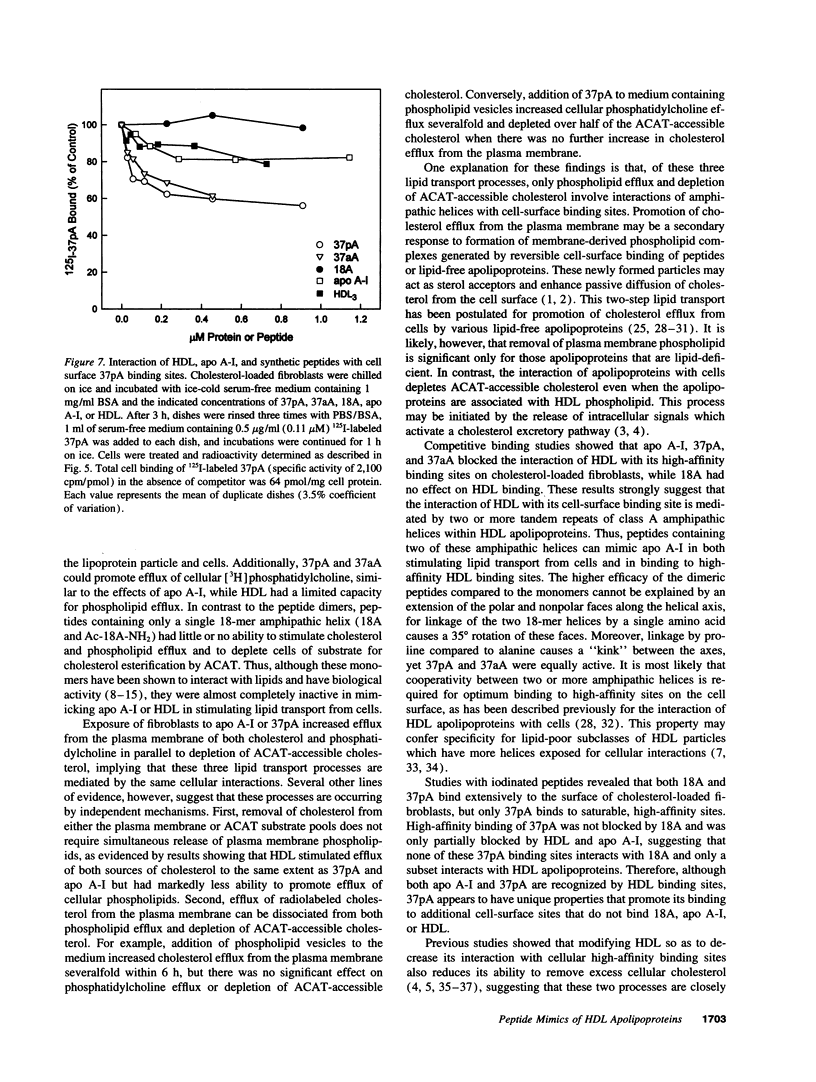
Clearance of excess cholesterol from cells by HDL is facilitated by the interaction of HDL apolipoproteins with cell-surface binding sites or receptors, a process that may be important in preventing atherosclerosis. In this study, synthetic peptides containing 18-mer amphipathic helices of the class found in HDL apolipoproteins (class A) were tested for their abilities to remove cholesterol and phospholipid from cultured sterol-laden fibroblasts and macrophages and to interact with cell-surface HDL binding sites. Lipid-free peptides containing two identical tandem repeats of class A amphipathic helices promoted cholesterol and phospholipid efflux from cells and depleted cellular cholesterol accessible for esterification by acyl CoA/cholesterol acyltransferase, similar to what was observed for purified apolipoprotein A-I. Peptide-mediated removal of plasma membrane cholesterol and depletion of acyl CoA/cholesterol acyltransferase-accessible cholesterol appeared to occur by separate mechanisms, as the latter process was less dependent on extracellular phospholipid. The dimeric amphipathic helical peptides also competed for high-affinity HDL binding sites on cholesterol-loaded fibroblasts and displayed saturable high-affinity binding to the cell surface. In contrast, peptides with a single helix had little or no ability to remove cellular cholesterol and phospholipid, or to interact with HDL binding sites, suggesting that cooperativity between two or more helical repeats is required for these activities. Thus, synthetic peptides comprising dimers of a structural motif common to exchangeable apolipoproteins can mimic apolipoprotein A-I in both binding to putative cell-surface receptors and clearing cholesterol from cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharamaiah G. M., Jones J. L., Brouillette C. G., Schmidt C. F., Chung B. H., Hughes T. A., Bhown A. S., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem. 1985 Aug 25;260(18):10248–10255. [PubMed] [Google Scholar]
- Bielicki J. K., Johnson W. J., Weinberg R. B., Glick J. M., Rothblat G. H. Efflux of lipid from fibroblasts to apolipoproteins: dependence on elevated levels of cellular unesterified cholesterol. J Lipid Res. 1992 Nov;33(11):1699–1709. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Blackburn W. D., Jr, Dohlman J. G., Venkatachalapathi Y. V., Pillion D. J., Koopman W. J., Segrest J. P., Anantharamaiah G. M. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. J Lipid Res. 1991 Dec;32(12):1911–1918. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Methods for studying the platelet-derived growth factor receptor. Methods Enzymol. 1985;109:69–100. doi: 10.1016/0076-6879(85)09078-4. [DOI] [PubMed] [Google Scholar]
- Brinton E. A., Oram J. F., Chen C. H., Albers J. J., Bierman E. L. Binding of high density lipoprotein to cultured fibroblasts after chemical alteration of apoprotein amino acid residues. J Biol Chem. 1986 Jan 5;261(1):495–503. [PubMed] [Google Scholar]
- Chung B. H., Anatharamaiah G. M., Brouillette C. G., Nishida T., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Correlation of structure with function. J Biol Chem. 1985 Aug 25;260(18):10256–10262. [PubMed] [Google Scholar]
- Duell P. B., Oram J. F., Bierman E. L. Nonenzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991 Mar;40(3):377–384. doi: 10.2337/diab.40.3.377. [DOI] [PubMed] [Google Scholar]
- Duell P. B., Oram J. F., Bierman E. L. Nonenzymatic glycosylation of HDL resulting in inhibition of high-affinity binding to cultured human fibroblasts. Diabetes. 1990 Oct;39(10):1257–1263. doi: 10.2337/diab.39.10.1257. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Gawish A., Iqbal M., Gupta K. B., Chen C. H., Segrest J. P., Anantharamaiah G. M. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charge distribution, hydrophobicity, and secondary structure on lipid association and lecithin:cholesterol acyltransferase activation. J Biol Chem. 1987 Jul 5;262(19):9389–9396. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Forte T. M., Goth-Goldstein R., Nordhausen R. W., McCall M. R. Apolipoprotein A-I-cell membrane interaction: extracellular assembly of heterogeneous nascent HDL particles. J Lipid Res. 1993 Feb;34(2):317–324. [PubMed] [Google Scholar]
- Garber D. W., Venkatachalapathi Y. V., Gupta K. B., Ibdah J., Phillips M. C., Hazelrig J. B., Segrest J. P., Anantharamaiah G. M. Turnover of synthetic class A amphipathic peptide analogues of exchangeable apolipoproteins in rats. Correlation with physical properties. Arterioscler Thromb. 1992 Aug;12(8):886–894. doi: 10.1161/01.atv.12.8.886. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Hara H., Komaba A., Yokoyama S. Alpha-helical requirements for free apolipoproteins to generate HDL and to induce cellular lipid efflux. Lipids. 1992 Apr;27(4):302–304. doi: 10.1007/BF02536480. [DOI] [PubMed] [Google Scholar]
- Hara H., Yokoyama S. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem. 1991 Feb 15;266(5):3080–3086. [PubMed] [Google Scholar]
- Hara H., Yokoyama S. Role of apolipoproteins in cholesterol efflux from macrophages to lipid microemulsion: proposal of a putative model for the pre-beta high-density lipoprotein pathway. Biochemistry. 1992 Feb 25;31(7):2040–2046. doi: 10.1021/bi00122a021. [DOI] [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Preparation, characterization, and measurement of lipoprotein lipase. Methods Enzymol. 1986;129:691–704. doi: 10.1016/0076-6879(86)29099-0. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Mahlberg F. H., Rothblat G. H., Phillips M. C. Cholesterol transport between cells and high-density lipoproteins. Biochim Biophys Acta. 1991 Oct 1;1085(3):273–298. doi: 10.1016/0005-2760(91)90132-2. [DOI] [PubMed] [Google Scholar]
- Jonas A., Steinmetz A., Churgay L. The number of amphipathic alpha-helical segments of apolipoproteins A-I, E, and A-IV determines the size and functional properties of their reconstituted lipoprotein particles. J Biol Chem. 1993 Jan 25;268(3):1596–1602. [PubMed] [Google Scholar]
- Jorgensen E. V., Anantharamaiah G. M., Segrest J. P., Gwynne J. T., Handwerger S. Synthetic amphipathic peptides resembling apolipoproteins stimulate the release of human placental lactogen. J Biol Chem. 1989 Jun 5;264(16):9215–9219. [PubMed] [Google Scholar]
- Kanellis P., Romans A. Y., Johnson B. J., Kercret H., Chiovetti R., Jr, Allen T. M., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charged amino acid residue topography on lipid affinity. J Biol Chem. 1980 Dec 10;255(23):11464–11472. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leblond L., Marcel Y. L. The amphipathic alpha-helical repeats of apolipoprotein A-I are responsible for binding of high density lipoproteins to HepG2 cells. J Biol Chem. 1991 Apr 5;266(10):6058–6067. [PubMed] [Google Scholar]
- Mendez A. J., Oram J. F., Bierman E. L. Protein kinase C as a mediator of high density lipoprotein receptor-dependent efflux of intracellular cholesterol. J Biol Chem. 1991 Jun 5;266(16):10104–10111. [PubMed] [Google Scholar]
- Oram J. F., Brinton E. A., Bierman E. L. Regulation of high density lipoprotein receptor activity in cultured human skin fibroblasts and human arterial smooth muscle cells. J Clin Invest. 1983 Nov;72(5):1611–1621. doi: 10.1172/JCI111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. F., Johnson C. J., Brown T. A. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. Evidence for reversible binding at the cell surface without internalization. J Biol Chem. 1987 Feb 15;262(5):2405–2410. [PubMed] [Google Scholar]
- Oram J. F., Mendez A. J., Slotte J. P., Johnson T. F. High density lipoprotein apolipoproteins mediate removal of sterol from intracellular pools but not from plasma membranes of cholesterol-loaded fibroblasts. Arterioscler Thromb. 1991 Mar-Apr;11(2):403–414. doi: 10.1161/01.atv.11.2.403. [DOI] [PubMed] [Google Scholar]
- Oram J. F. Receptor-mediated transport of cholesterol between cultured cells and high-density lipoproteins. Methods Enzymol. 1986;129:645–659. doi: 10.1016/0076-6879(86)29096-5. [DOI] [PubMed] [Google Scholar]
- Owens B. J., Anantharamaiah G. M., Kahlon J. B., Srinivas R. V., Compans R. W., Segrest J. P. Apolipoprotein A-I and its amphipathic helix peptide analogues inhibit human immunodeficiency virus-induced syncytium formation. J Clin Invest. 1990 Oct;86(4):1142–1150. doi: 10.1172/JCI114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Johnson W. J., Rothblat G. H. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987 Jun 24;906(2):223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Mahlberg F. H., Johnson W. J., Phillips M. C. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J Lipid Res. 1992 Aug;33(8):1091–1097. [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992 Feb;33(2):141–166. [PubMed] [Google Scholar]
- Theret N., Delbart C., Aguie G., Fruchart J. C., Vassaux G., Ailhaud G. Cholesterol efflux from adipose cells is coupled to diacylglycerol production and protein kinase C activation. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1361–1368. doi: 10.1016/s0006-291x(05)80938-6. [DOI] [PubMed] [Google Scholar]
- Venkatachalapathi Y. V., Phillips M. C., Epand R. M., Epand R. F., Tytler E. M., Segrest J. P., Anantharamaiah G. M. Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins. 1993 Apr;15(4):349–359. doi: 10.1002/prot.340150403. [DOI] [PubMed] [Google Scholar]


